Содержание
- 2. LECTURE No. 3 CARBON BASED MATERIALS
- 3. Introduction Carbon is a well-known chemical element. It is present in many areas of our life.
- 4. OBJECTIVES To describe carbon nanotubes, graphene and nanoporous materials. To explain properties of carbon based materials
- 5. OUTLINE Carbon nanotubes. Characteristics, types and applications. Graphene. Structure and properties. Porous carbon materials. Characteristics, sources
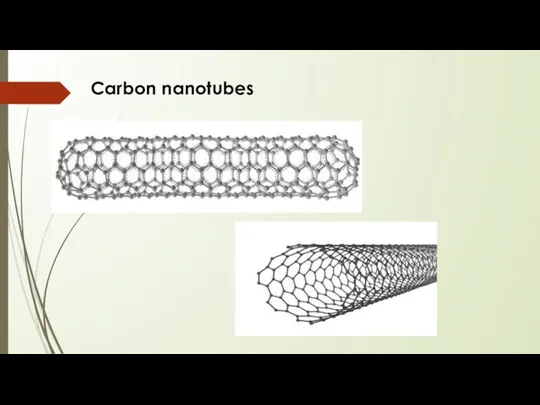
- 6. Carbon nanotubes Tubular structures whose diameter is of the order of nanometer (nm) Length of several
- 7. Carbon nanotubes
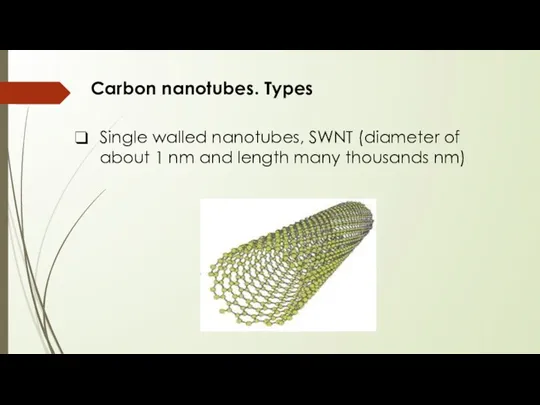
- 8. Carbon nanotubes. Types Single walled nanotubes, SWNT (diameter of about 1 nm and length many thousands
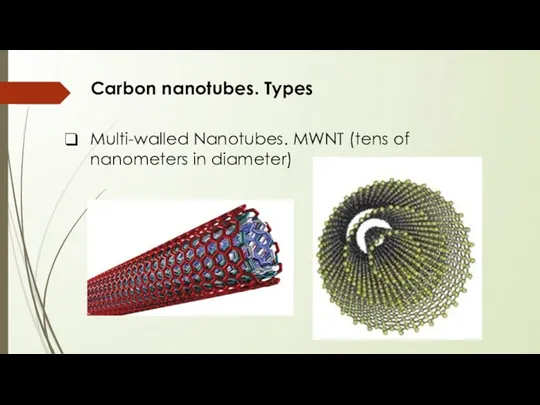
- 9. Carbon nanotubes. Types Multi-walled Nanotubes. MWNT (tens of nanometers in diameter)
- 10. Carbon nanotubes. Characteristics 100 thousand times thinner than a human hair, but it is a very
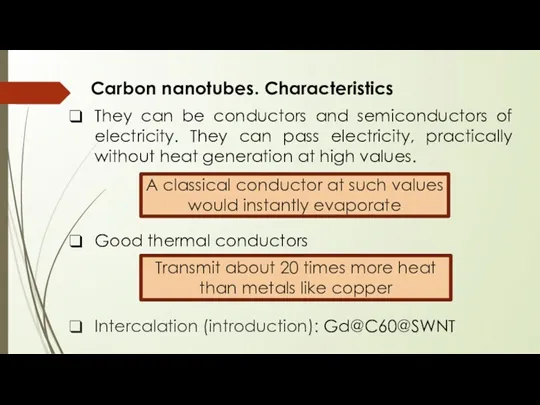
- 11. Carbon nanotubes. Characteristics They can be conductors and semiconductors of electricity. They can pass electricity, practically
- 12. Applications Semiconductor heterostructures, i.e. structures such as "metal / semiconductor" Television and computer screens Needle for
- 13. Graphene Graphene was opened in 2004 by A. Geim and K. Novoselov. For the discovery of
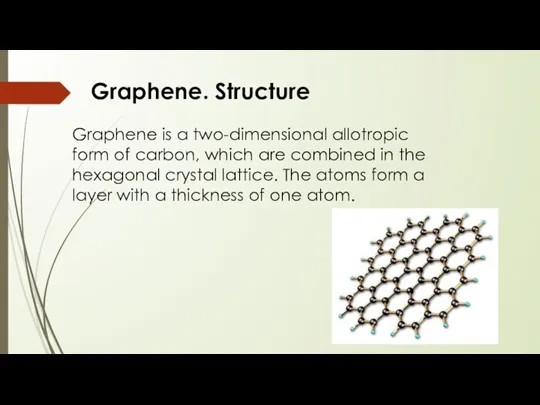
- 14. Graphene is a two-dimensional allotropic form of carbon, which are combined in the hexagonal crystal lattice.
- 15. Properties of Graphene High strength. Its sheet with area of one square meter and a thickness
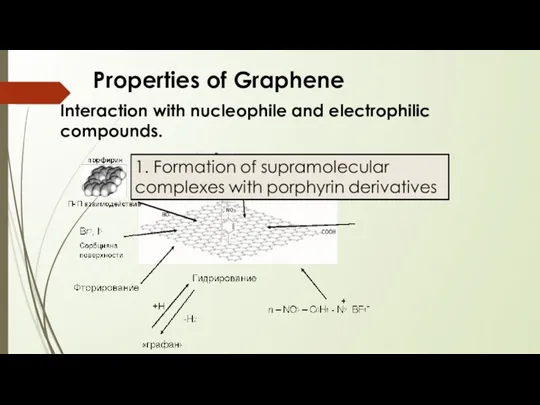
- 16. Properties of Graphene Interaction with nucleophile and electrophilic compounds.
- 17. Properties of Graphene Interaction with nucleophile and electrophilic compounds.
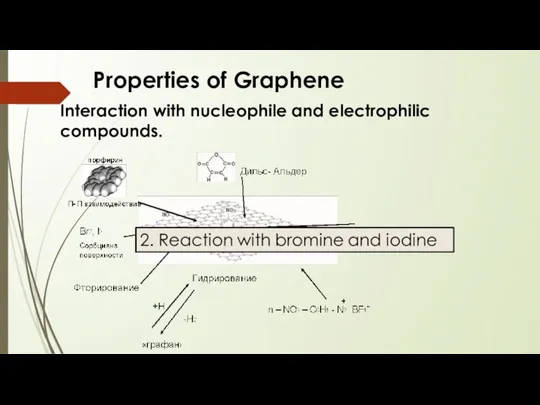
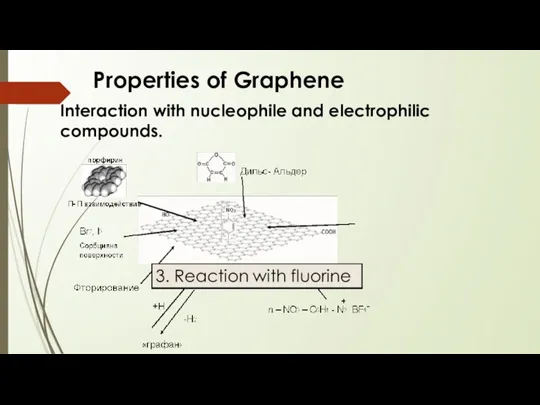
- 18. Properties of Graphene Interaction with nucleophile and electrophilic compounds.
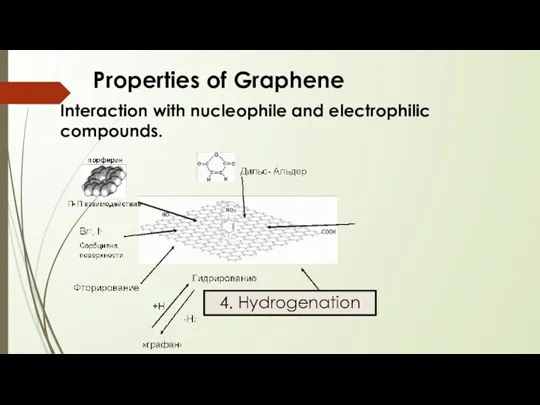
- 19. Properties of Graphene Interaction with nucleophile and electrophilic compounds. 4. Hydrogenation
- 20. Porous carbon materials. Features High specific adsorption and catalytic activity. Stability in non-oxidative media. Possibility of
- 21. Porous carbon materials. Sources for production. wood, stone and brown coals, agricultural waste, polymeric materials, liquid
- 22. Porous carbon materials. Production. Physical activation - preparation of raw materials (separation, crushing, drying) - pyrolysis
- 23. Porous carbon materials. Production. Thermochemical activation - introduction of chemical additives into the starting material. -
- 24. Porous carbon materials. Considerations for their use. Specific surface area, Pore volume and size, Pore size
- 25. Porous carbon materials. Chemical modifications. Anthracites: insufficient and inefficient (temperature, reagent ratio, reaction medium). Brown coals:
- 26. Porous carbon materials. Applications Catalysts Adsorbents for chromatography Gas storage systems Environmental protection
- 27. Поскольку пористые углеродные материалы получают из любого вида углеродсодержащего сырья, включая отходы, и сами применяются в
- 28. Control questions 1. Describe, briefly, the structure of carbon nanotubes. Mention existing types. 2. Explain some
- 30. Скачать презентацию

















 Классификация оптических методов анализа. Абсорбционная молекулярная спектроскопия
Классификация оптических методов анализа. Абсорбционная молекулярная спектроскопия Структурные схемы
Структурные схемы Свободное падение тел. Движение с ускорением свободного падения
Свободное падение тел. Движение с ускорением свободного падения Межатомные взаимодействия в конденсированных средах
Межатомные взаимодействия в конденсированных средах Механикалық гармониялық тербелістер. Өшетін тербелістер. Еріксіз тербелістер. Толқындар
Механикалық гармониялық тербелістер. Өшетін тербелістер. Еріксіз тербелістер. Толқындар Оборудование для ремонта бытовых электроприборов
Оборудование для ремонта бытовых электроприборов Магнитные свойства аморфных микропроводов с положительной магнитострикцией
Магнитные свойства аморфных микропроводов с положительной магнитострикцией Изотопы. Радиоактивные превращения атомных ядер
Изотопы. Радиоактивные превращения атомных ядер Физическая природа радуги. Создание радуги в домашних условиях
Физическая природа радуги. Создание радуги в домашних условиях Оптические приборы, вооружающие глаз
Оптические приборы, вооружающие глаз Сверхпроводимость II рода. Криогенные и сверхпроводящие электроэнергетические устройства. Лекция 3
Сверхпроводимость II рода. Криогенные и сверхпроводящие электроэнергетические устройства. Лекция 3 Работа и мощность
Работа и мощность Постоянный электрический ток
Постоянный электрический ток Использование ФЦИОР на уроках фиизики Диск
Использование ФЦИОР на уроках фиизики Диск Работа и энергия
Работа и энергия Температура. Единицы изменения температуры
Температура. Единицы изменения температуры Электропривод и его функциональная схема. Введение в дисциплину
Электропривод и его функциональная схема. Введение в дисциплину Звуковой резонанс
Звуковой резонанс Изнашивание неметаллических материалов
Изнашивание неметаллических материалов Деформації. Сили пружності
Деформації. Сили пружності Теория турбомашин. Введение. Задачи курса, его метод и связь с другими предметами учебного плана
Теория турбомашин. Введение. Задачи курса, его метод и связь с другими предметами учебного плана Презентация Силы в природе
Презентация Силы в природе Генератор переменного тока ( устаревшее альтернатор)
Генератор переменного тока ( устаревшее альтернатор) Интерактивная мозаика-2017. Знатоки физики 8 класс
Интерактивная мозаика-2017. Знатоки физики 8 класс Защита от ионизирующих излучений
Защита от ионизирующих излучений Геофизические исследования скважин (ГИС). Методы каротажа
Геофизические исследования скважин (ГИС). Методы каротажа Электрооборудование автомобилей. Автоматическая коробка переключения передач с электронным управлением
Электрооборудование автомобилей. Автоматическая коробка переключения передач с электронным управлением Сила тока. Условия, необходимые для существования электрического тока. Закон Ома для участка цепи
Сила тока. Условия, необходимые для существования электрического тока. Закон Ома для участка цепи