Содержание
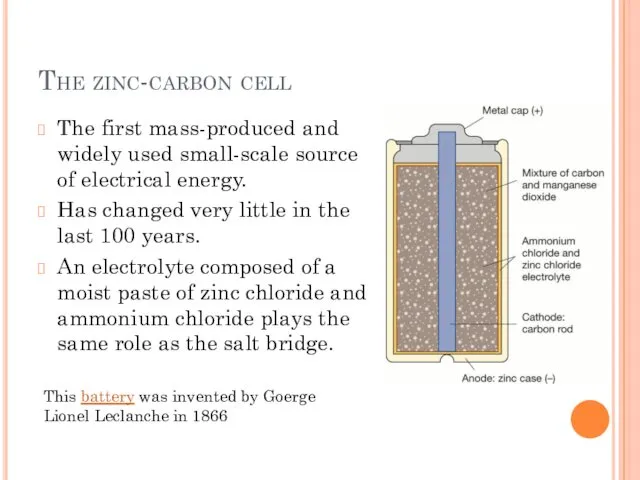
- 2. The zinc-carbon cell The first mass-produced and widely used small-scale source of electrical energy. Has changed
- 3. The zinc-carbon cell At the anode (-) oxidation of the zinc case produces electrons: Zn(s) →
- 4. The zinc-carbon dry cell A new cell produces about 1.5 volts, but this diminishes significantly during
- 5. Voltage Rating of Zinc Carbon Battery Standard voltage rating of a zinc carbon battery is determined
- 6. Advantages and Disadvantages of Zinc Carbon Battery Advantages of Leclanche’ Battery The cost of this battery
- 7. Alkaline batteries Alkaline batteries and alkaline cells (a battery being a collection of multiple cells) are
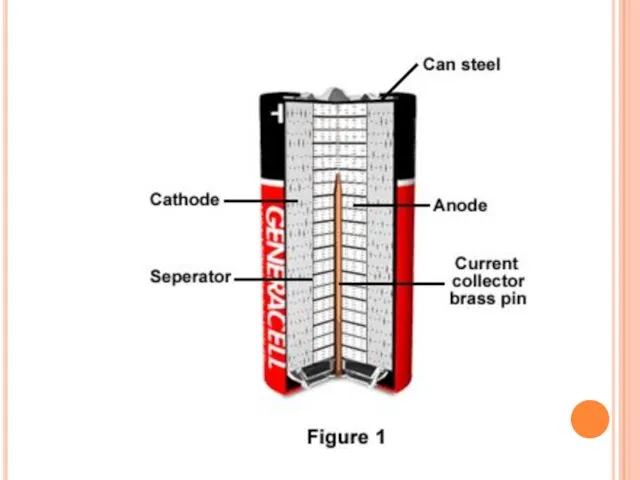
- 8. Construction A cylindrical cell is contained in a drawn steel can, which is the cathode current
- 10. Chemistry Anode : Zinc Powder Cathode : Manganese dioxide(MnO2) powder Electrolyte : Potassium hydroxide(KOH)
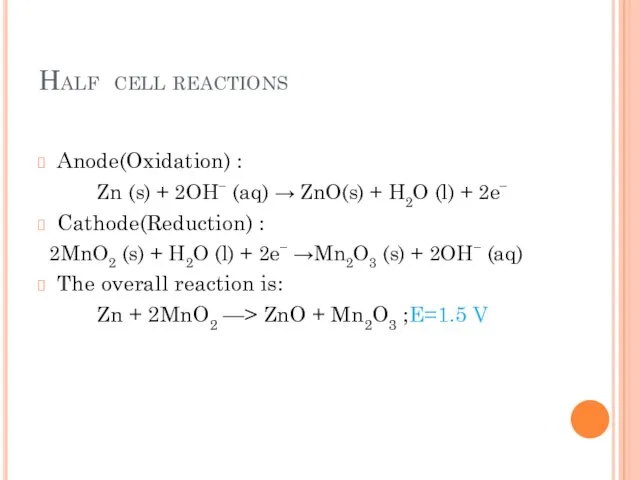
- 11. Half cell reactions Anode(Oxidation) : Zn (s) + 2OH− (aq) → ZnO(s) + H2O (l) +
- 12. Advantages Better low temperature performance than zinc carbon. Continue to function in sub-zero temperatures. Less leakage
- 14. Скачать презентацию








 Тасымалдау құбылысы. Нақты газдар
Тасымалдау құбылысы. Нақты газдар История создания электрической лампы
История создания электрической лампы Електричний струм у металах, рідинах і газах. (Лекція 11)
Електричний струм у металах, рідинах і газах. (Лекція 11) Стартовая презентация к проекту Зрение и компьютер
Стартовая презентация к проекту Зрение и компьютер Громкость звука. 9 класс
Громкость звука. 9 класс Реализация компетентностного подхода как условие повышения качества образования по физике
Реализация компетентностного подхода как условие повышения качества образования по физике Основные положения по организации и технологии войскового ремонта машин
Основные положения по организации и технологии войскового ремонта машин Ультразвуковые колебания. Ультразвук и инфразвук
Ультразвуковые колебания. Ультразвук и инфразвук презентация на тему Сообщающиеся сосуды
презентация на тему Сообщающиеся сосуды Физика в системе естественных наук. Кинематика поступательного движения. (Лекция 1)
Физика в системе естественных наук. Кинематика поступательного движения. (Лекция 1) презентация к уроку на тему: Основные положения МКТ
презентация к уроку на тему: Основные положения МКТ презентацияВоздухоплавание
презентацияВоздухоплавание ЖРД. Устройство и принцип действия, внутрикамерные процессы. (Лекция 2)
ЖРД. Устройство и принцип действия, внутрикамерные процессы. (Лекция 2) Основные параметры и характеристики передающих антенн
Основные параметры и характеристики передающих антенн Сложное движение твердого тела
Сложное движение твердого тела Повторение по теме Электромагнитная индукция
Повторение по теме Электромагнитная индукция Открытие электрической лампочки
Открытие электрической лампочки Виды излучений
Виды излучений Electric Vehicles 101
Electric Vehicles 101 Сұйықтар мен газдар механикасының негізгі ұғымдары
Сұйықтар мен газдар механикасының негізгі ұғымдары Движение заряженной частицы в электрическом и магнитном полях. Лекция 6
Движение заряженной частицы в электрическом и магнитном полях. Лекция 6 Техника изготовления и особенности конструирования съемных ортодонтических аппаратов
Техника изготовления и особенности конструирования съемных ортодонтических аппаратов Измерение размеров малых тел. Взаимное притяжение молекул
Измерение размеров малых тел. Взаимное притяжение молекул Электротехника и электроника
Электротехника и электроника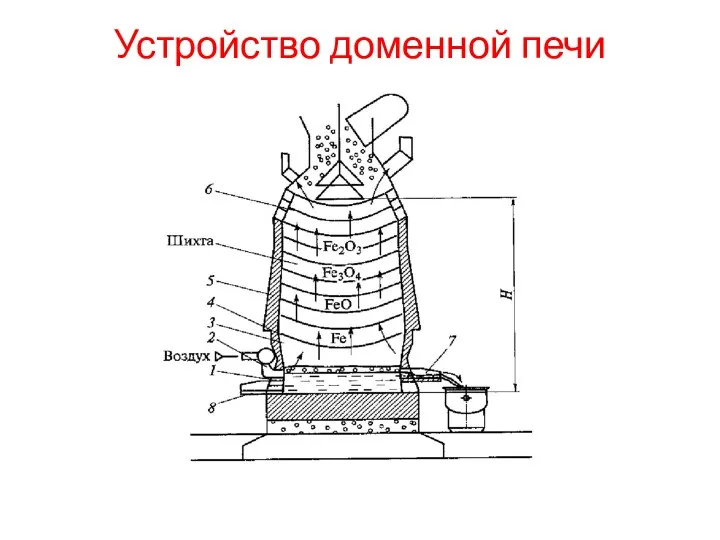 Устройство доменной печи. (Лекция 4)
Устройство доменной печи. (Лекция 4) Линзы. Оптическая сила линзы
Линзы. Оптическая сила линзы Линзы. Оптическая сила линзы
Линзы. Оптическая сила линзы Of steering
Of steering