Hprogram of subject (syllabus). Recent developments of biotechnology in veterinary medicine and animal husbandry презентация
Содержание
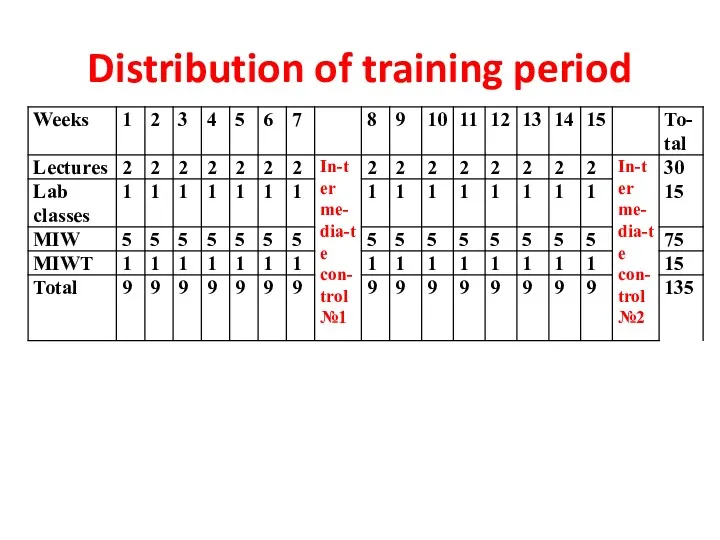
- 2. Distribution of training period
- 3. Course objectives is: to familiarize Masters with new developments and achievements of modern Biotechnology in the
- 4. As a result of studying this subject, masters must know: the latest achievements of world science
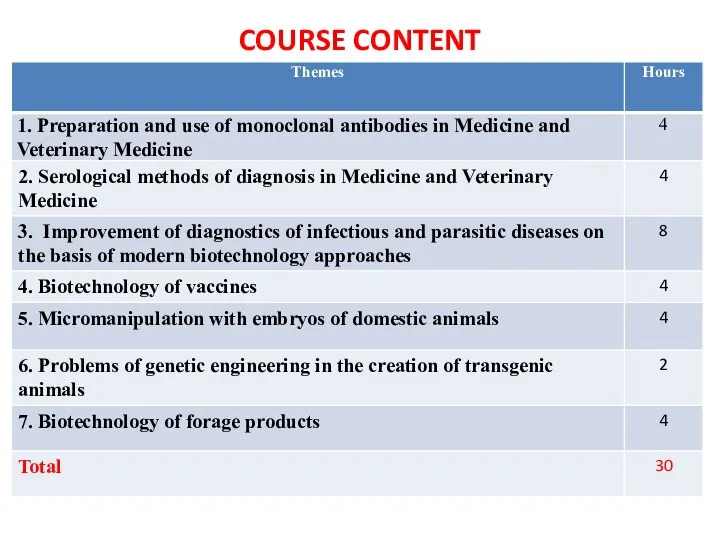
- 5. COURSE CONTENT
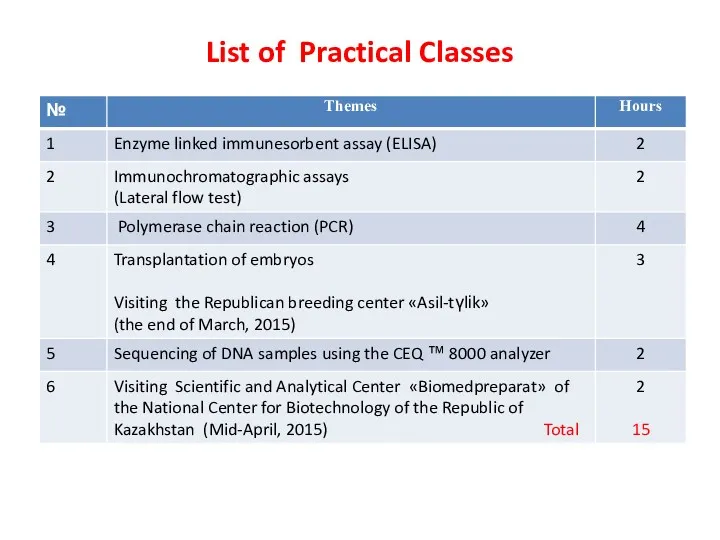
- 6. List of Practical Classes
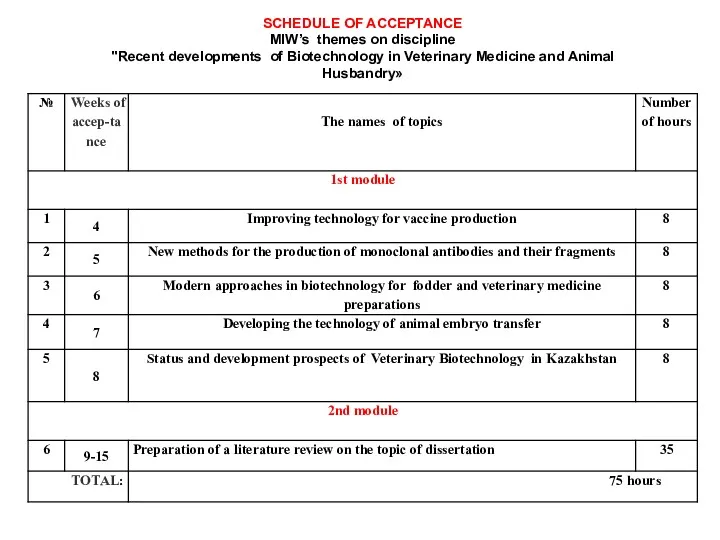
- 7. SCHEDULE OF ACCEPTANCE MIW’s themes on discipline "Recent developments of Biotechnology in Veterinary Medicine and Animal
- 8. REFERENCE Basic Literature: The Basic literature of the discipline are articles and reviews published in scientific
- 9. HYBRIDOMA TECHNIQUE TEACHING OBJECTIVES: 1.INTRODUCTION 2. PRINCIPLE INVOLVED IN MONOCLONAL ANTIBODIES PRODUCTION 3. PRODUCTION OF MONOCLONAL
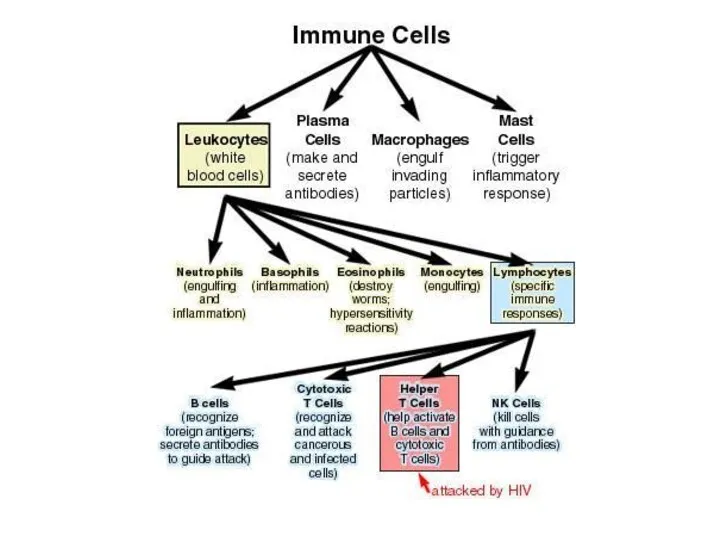
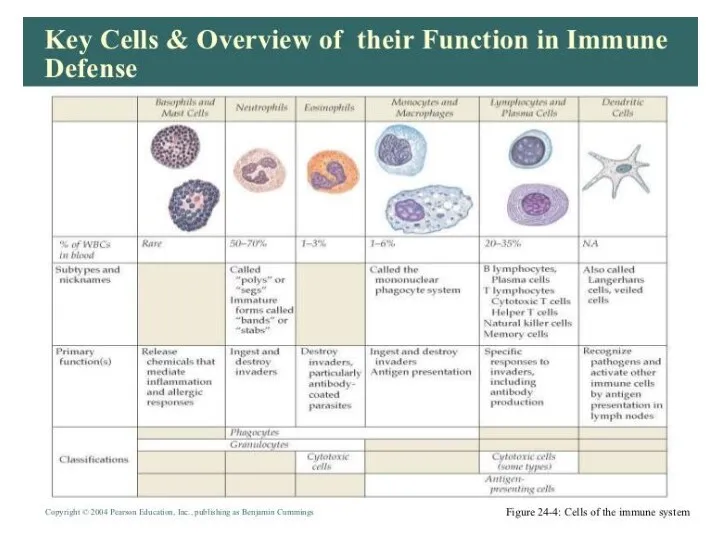
- 10. INTRODUCTION
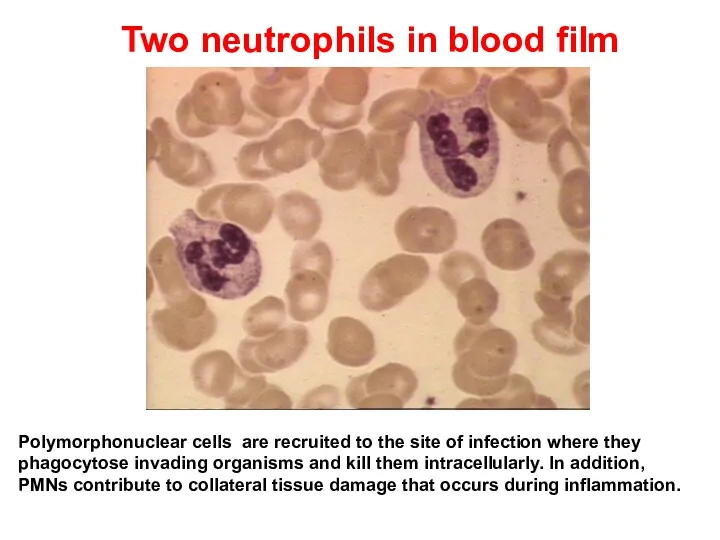
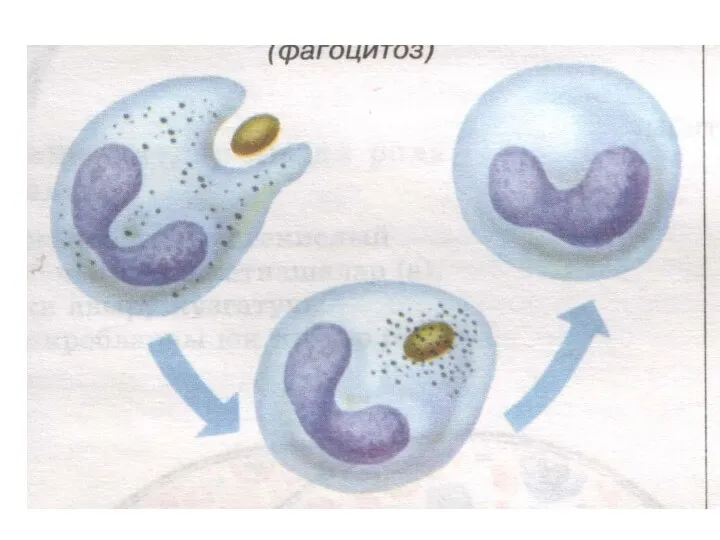
- 13. Two neutrophils in blood film Polymorphonuclear cells are recruited to the site of infection where they
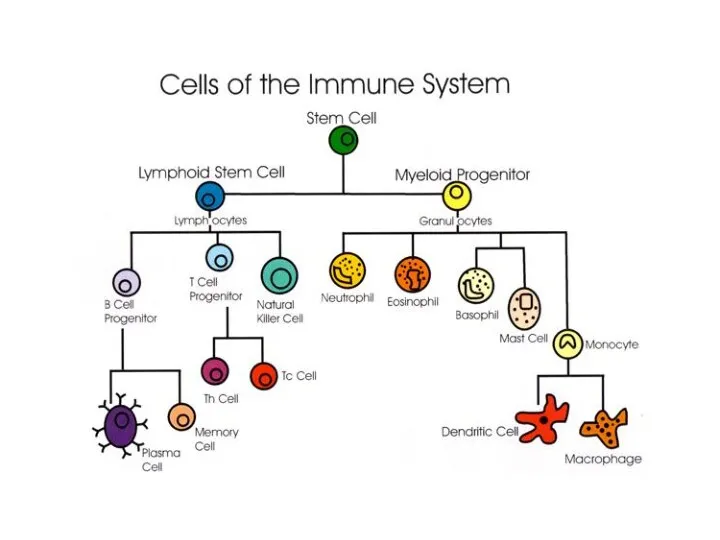
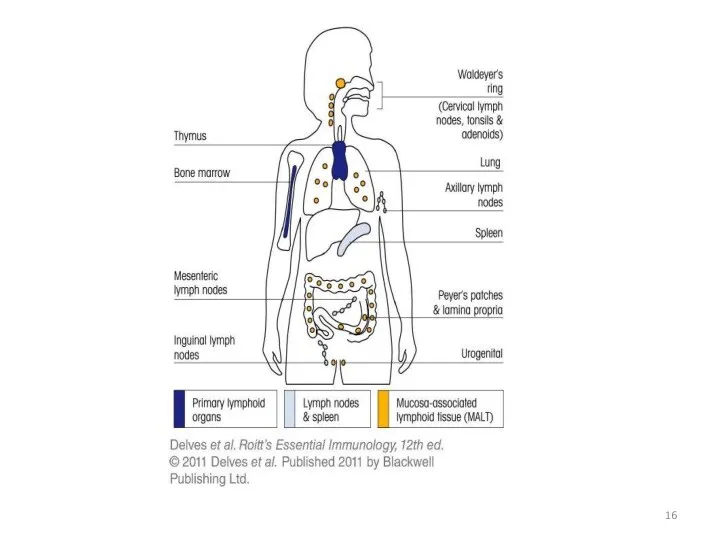
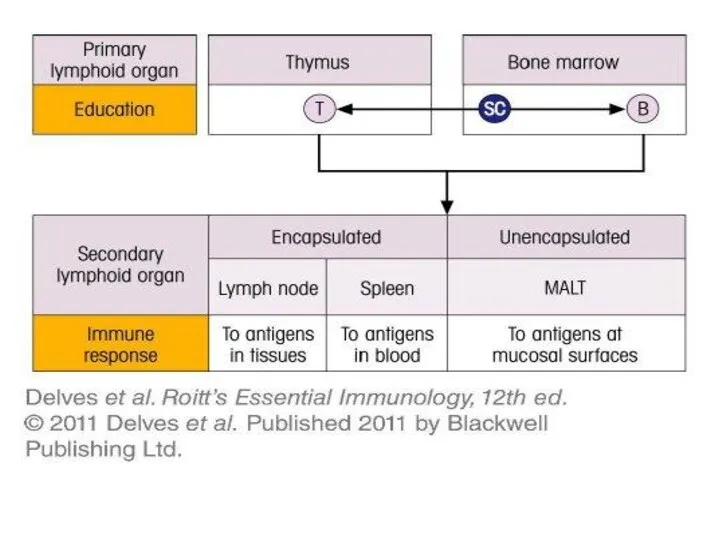
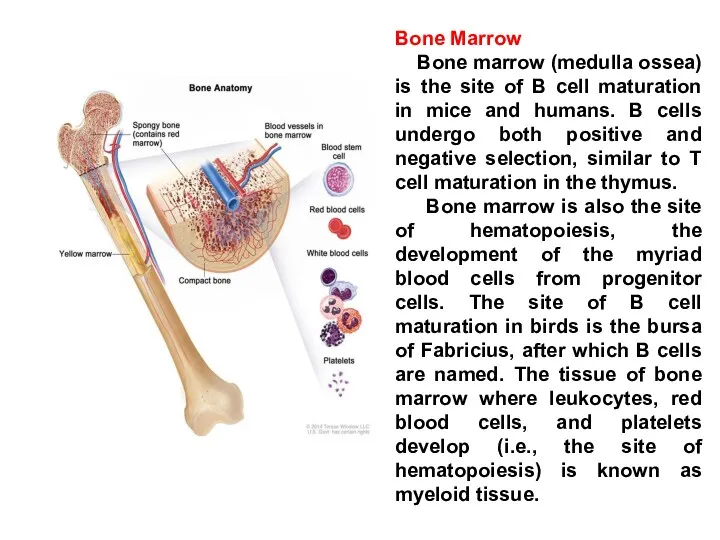
- 18. Bone Marrow Bone marrow (medulla ossea) is the site of B cell maturation in mice and
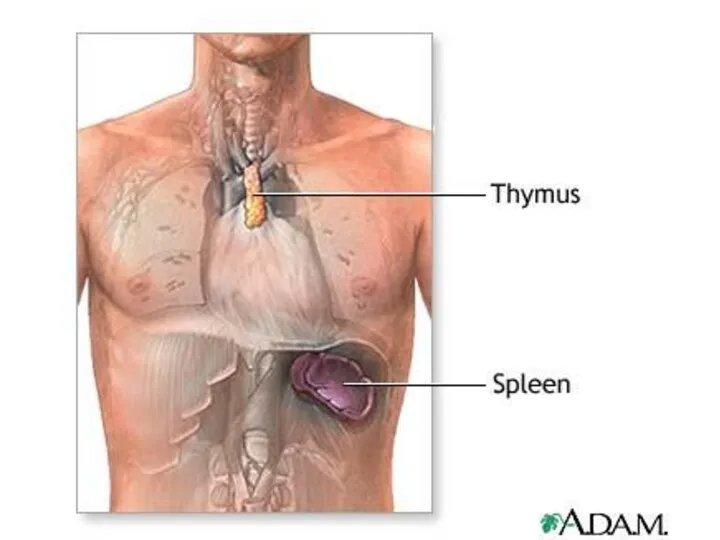
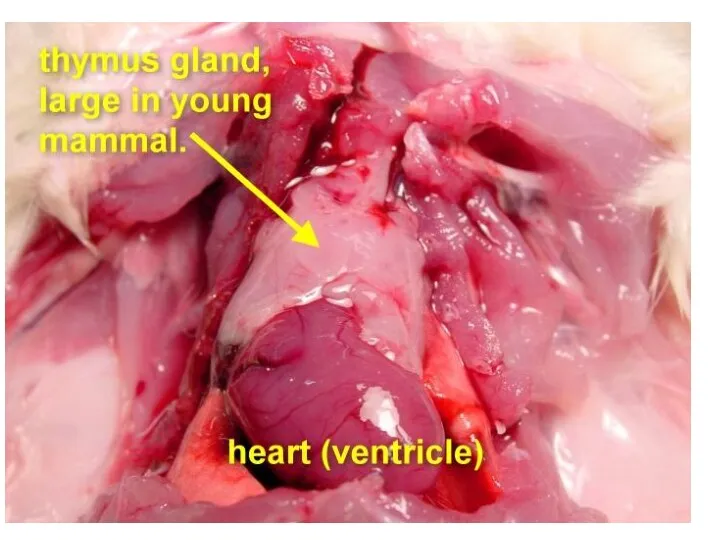
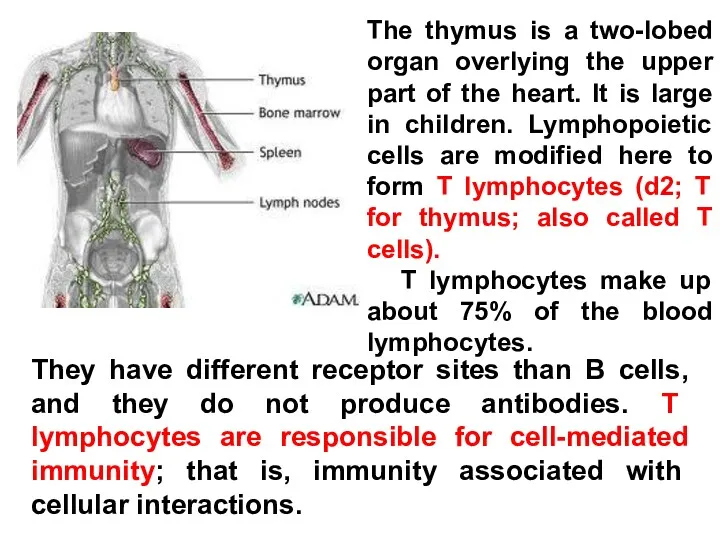
- 21. The thymus is a two-lobed organ overlying the upper part of the heart. It is large
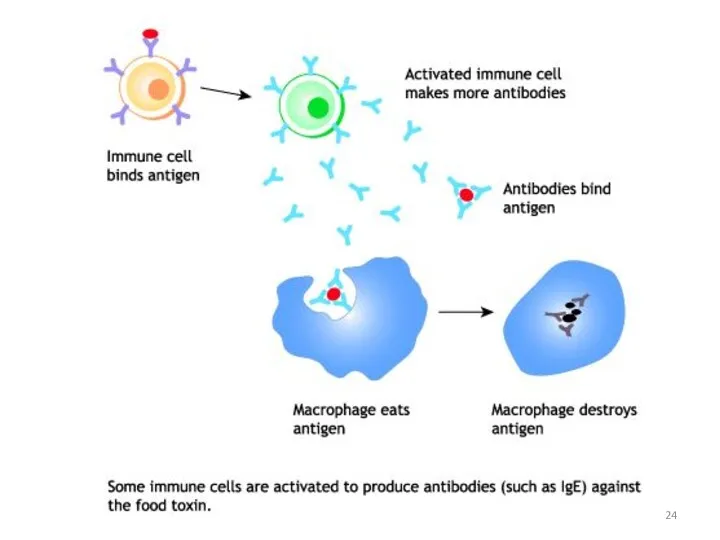
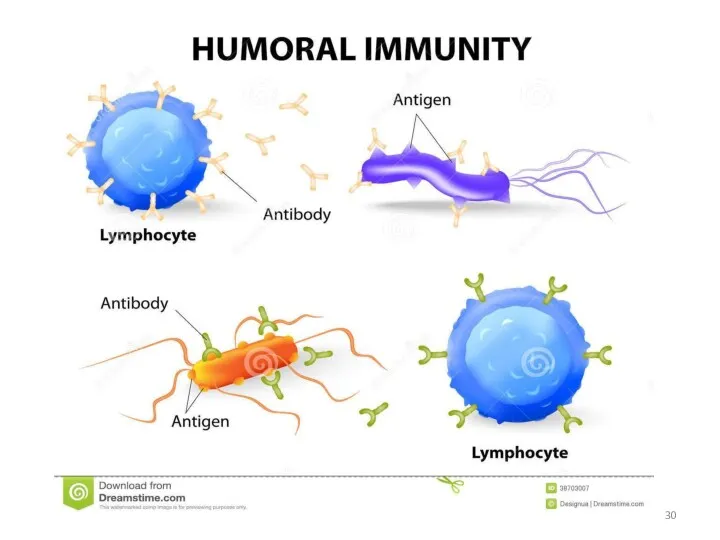
- 25. Antibodies are produced by a specialized group of cells called B-Lymphocytes. When an foreign antigen enters
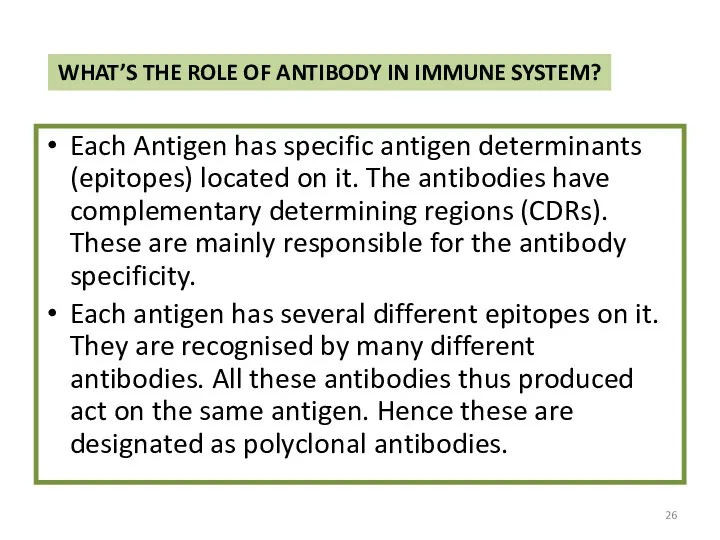
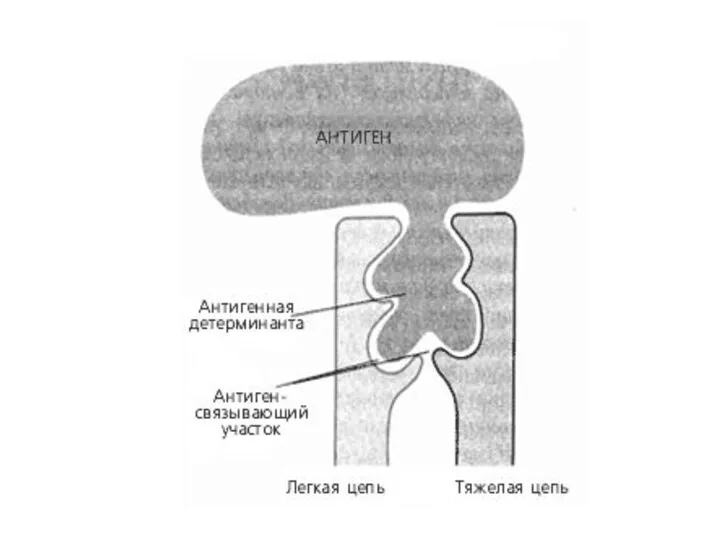
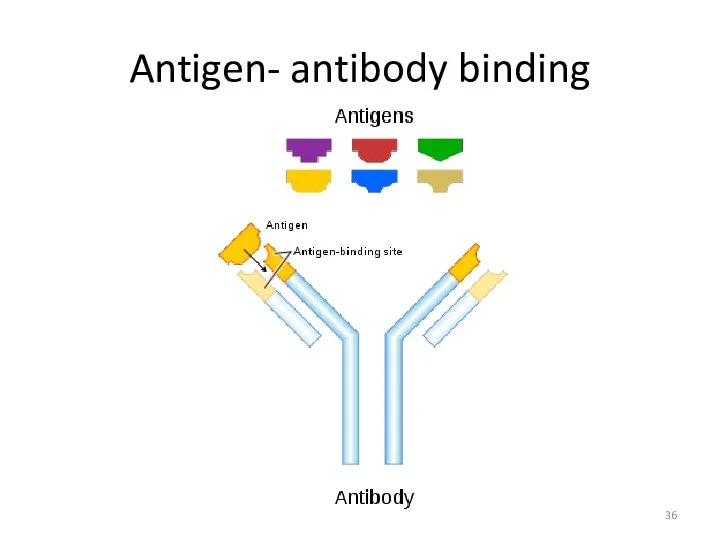
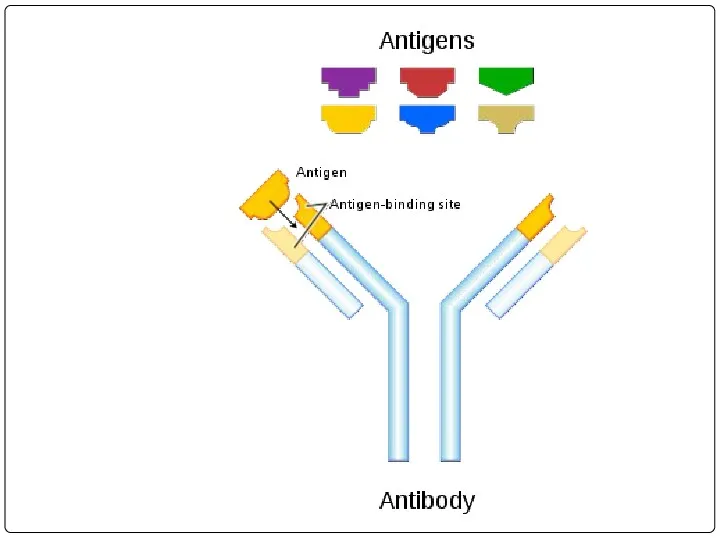
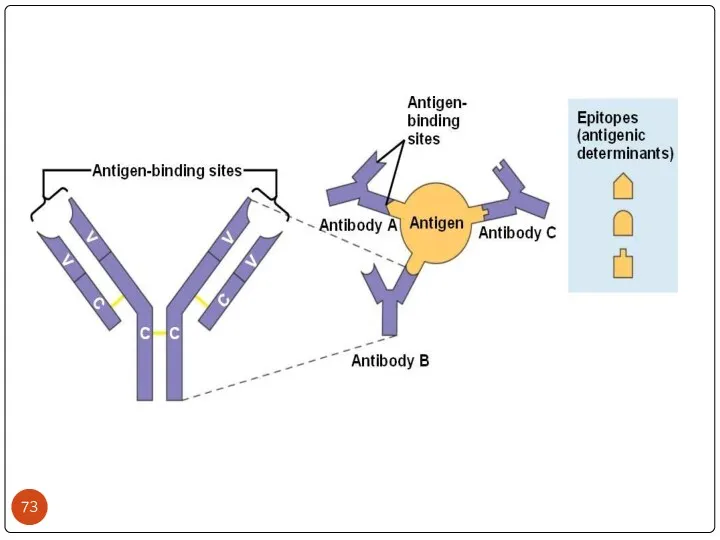
- 26. WHAT’S THE ROLE OF ANTIBODY IN IMMUNE SYSTEM? Each Antigen has specific antigen determinants (epitopes) located
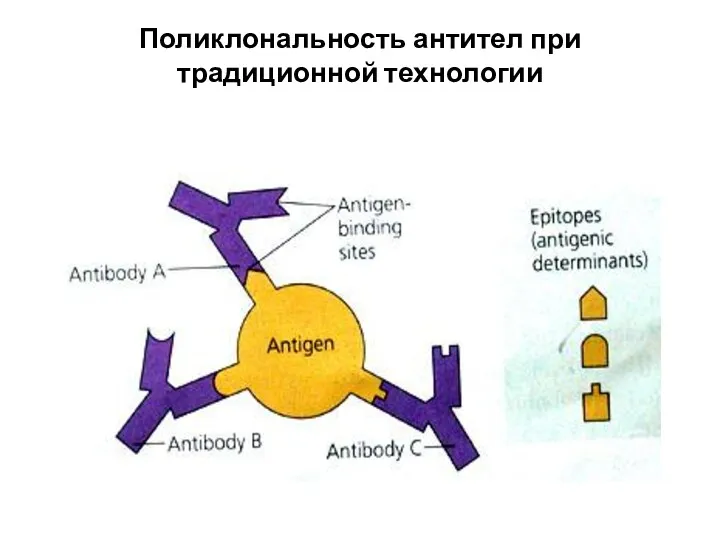
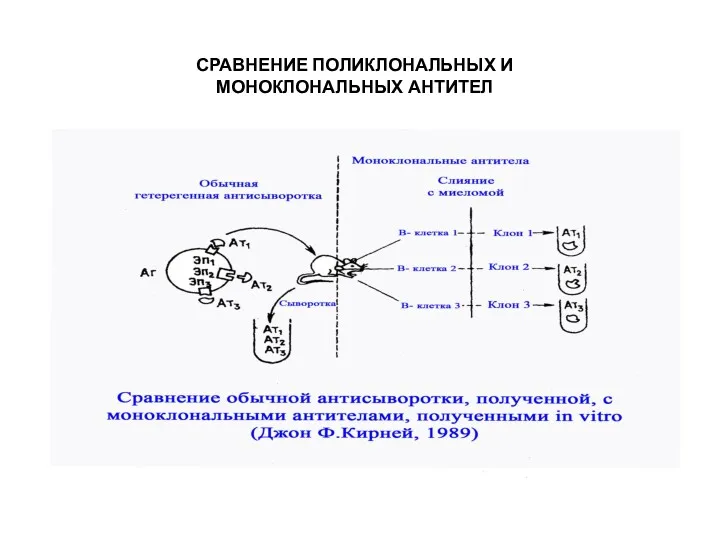
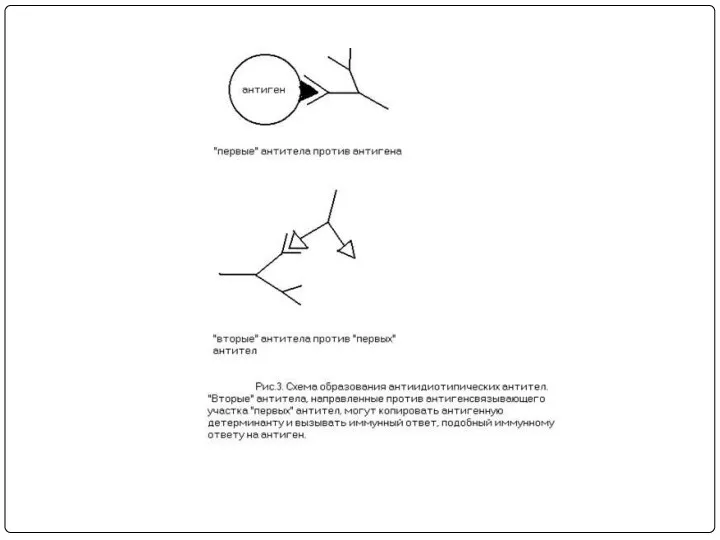
- 27. Поликлональность антител при традиционной технологии
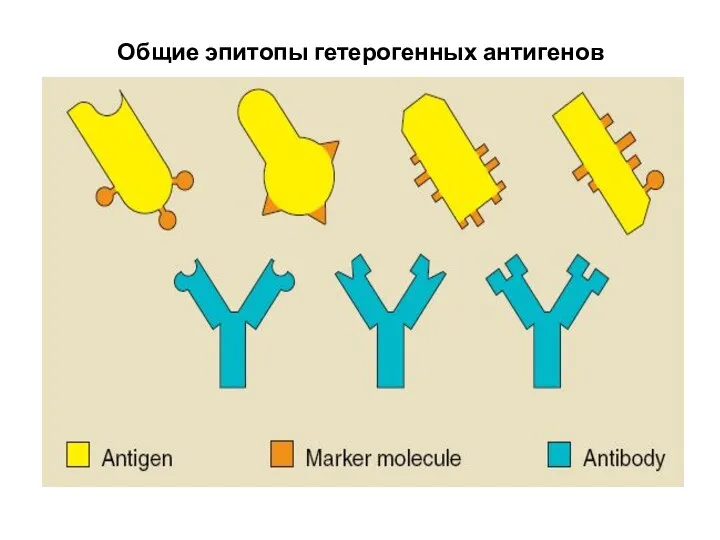
- 28. Общие эпитопы гетерогенных антигенов
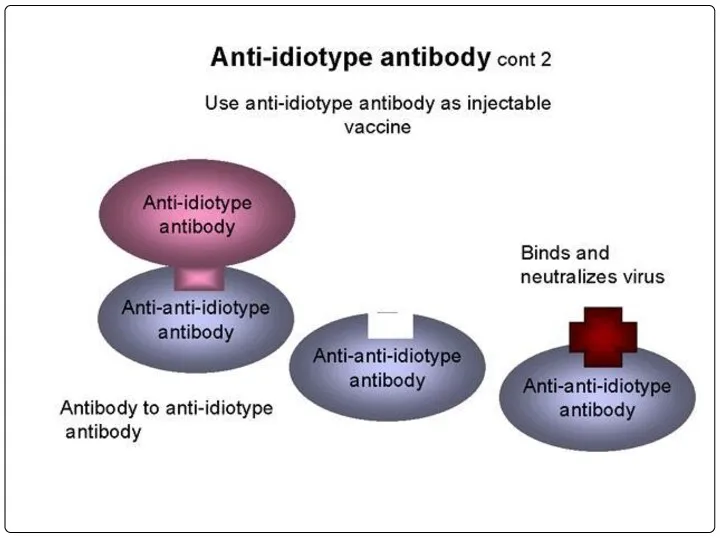
- 31. WHAT’S THE NEED TO DEVELOP MONOCLONAL ANTIBODIES? In general naturally produced antibodies are non-specific and heterogenous
- 32. WHAT ARE MONOCLONAL ANTIBODIES? MAb is a single type of antibody that is directed against a
- 33. СРАВНЕНИЕ ПОЛИКЛОНАЛЬНЫХ И МОНОКЛОНАЛЬНЫХ АНТИТЕЛ
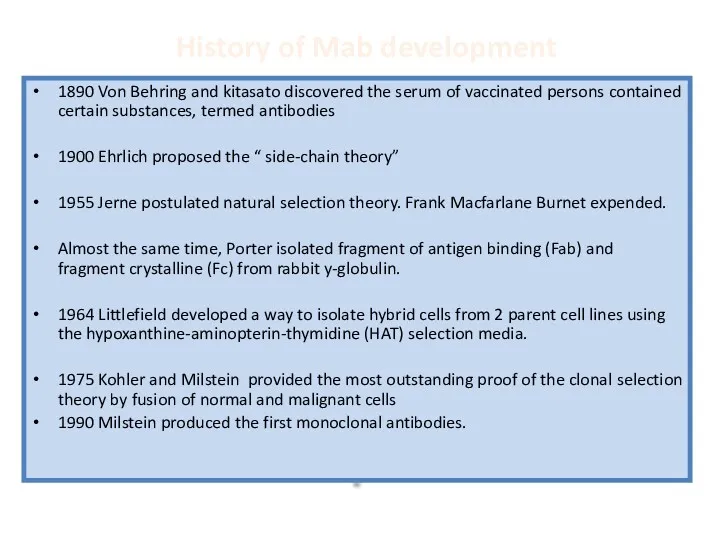
- 34. History of Mab development 1890 Von Behring and kitasato discovered the serum of vaccinated persons contained
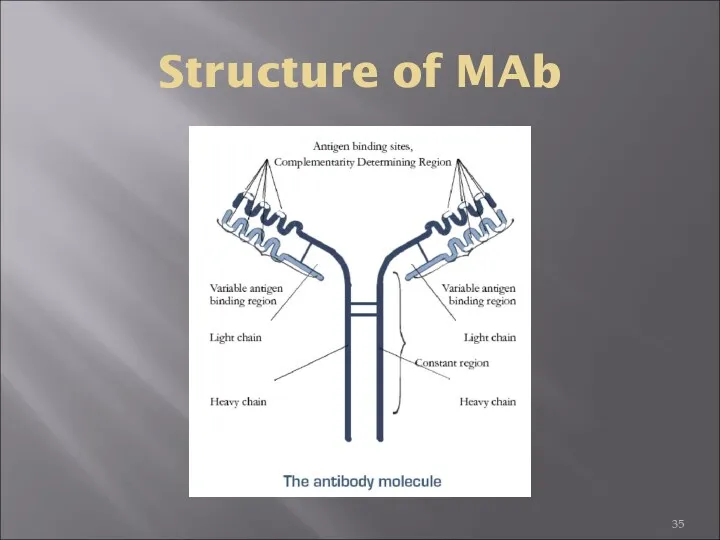
- 35. Structure of MAb
- 36. Antigen- antibody binding
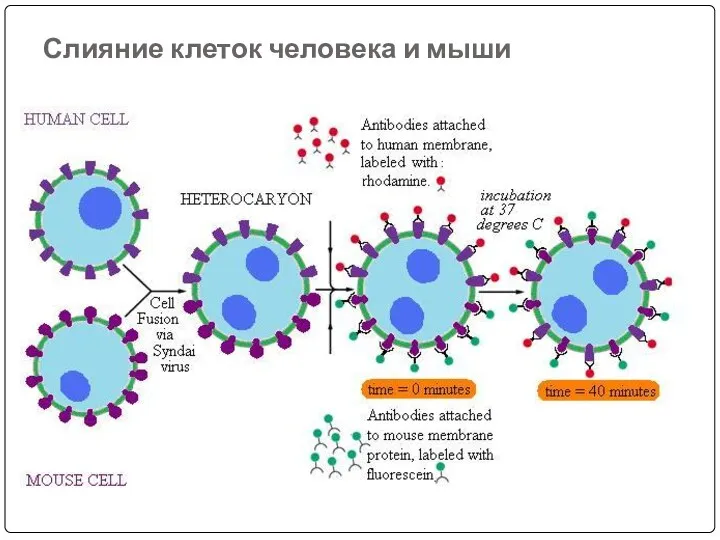
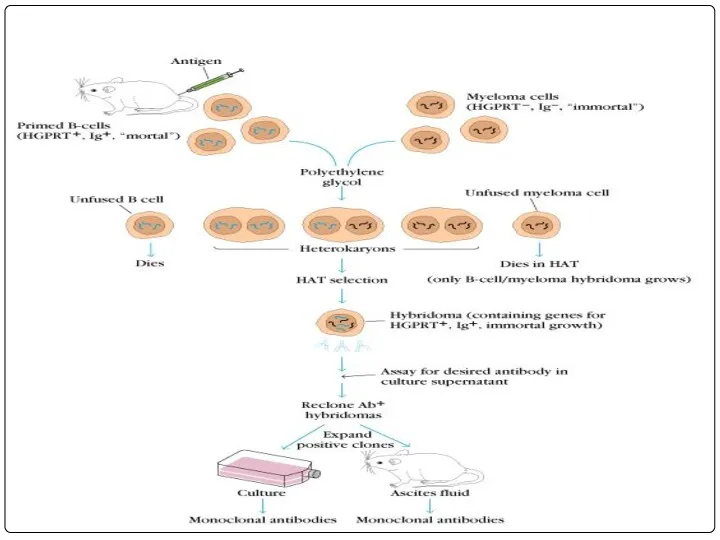
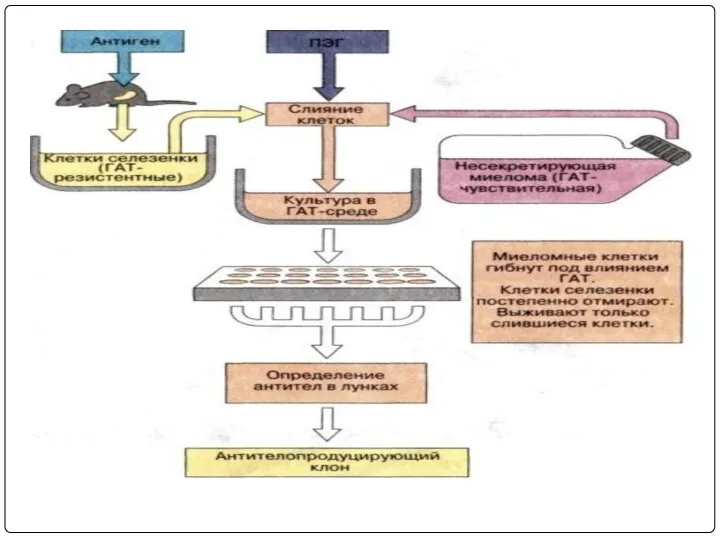
- 37. Hybridoma technology: In this B-Lymphocytes and myeloma cells are mixed together and exposed to PEG for
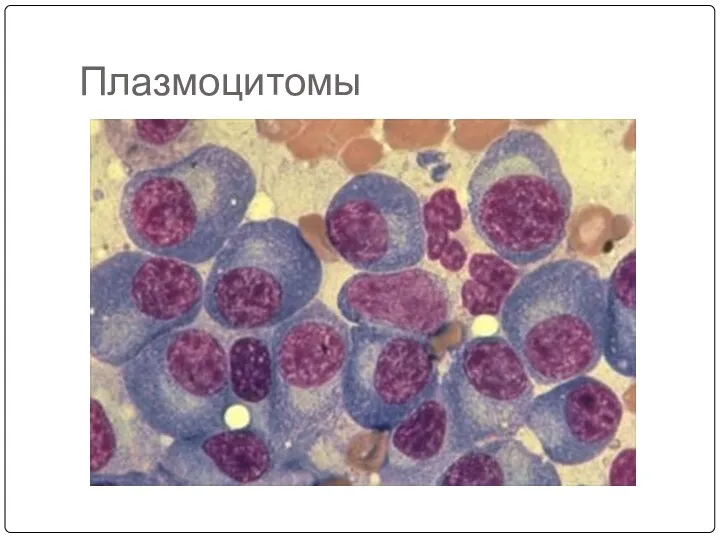
- 38. Плазмоцитомы
- 39. Слияние клеток человека и мыши
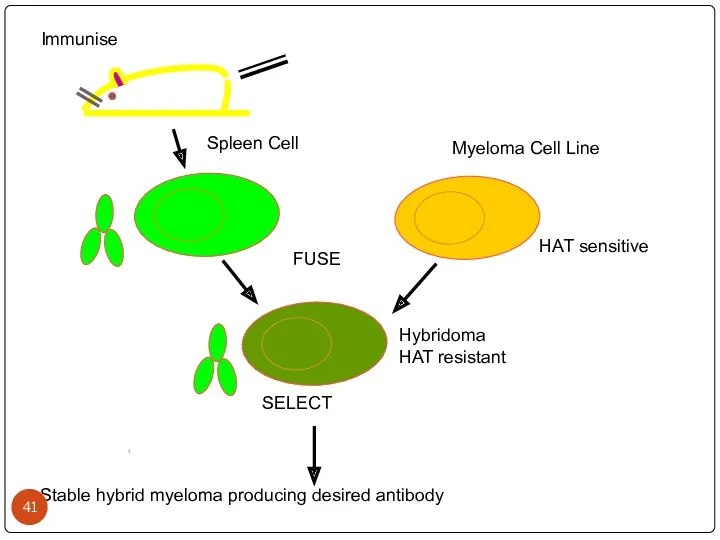
- 41. Immunise Spleen Cell Myeloma Cell Line FUSE HAT sensitive Hybridoma HAT resistant Stable hybrid myeloma producing
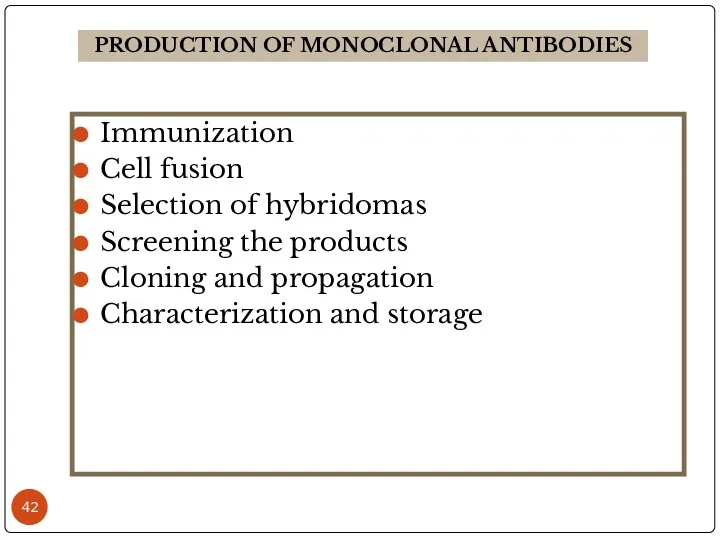
- 42. Immunization Cell fusion Selection of hybridomas Screening the products Cloning and propagation Characterization and storage PRODUCTION
- 43. Мыши линии Balb/c
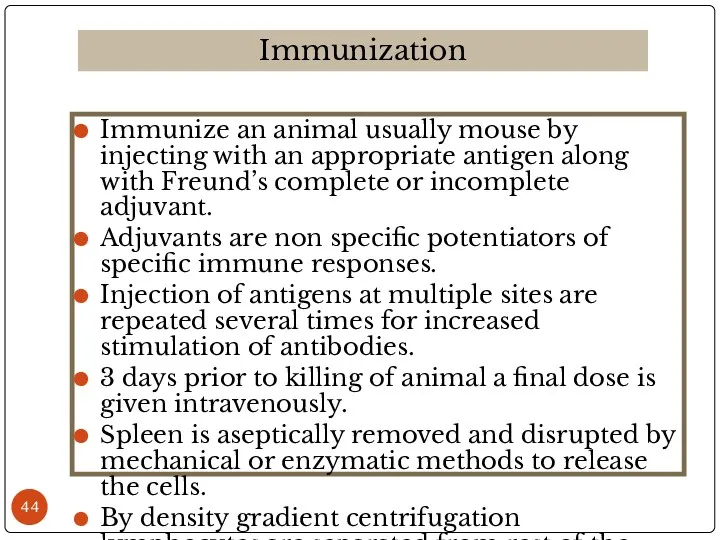
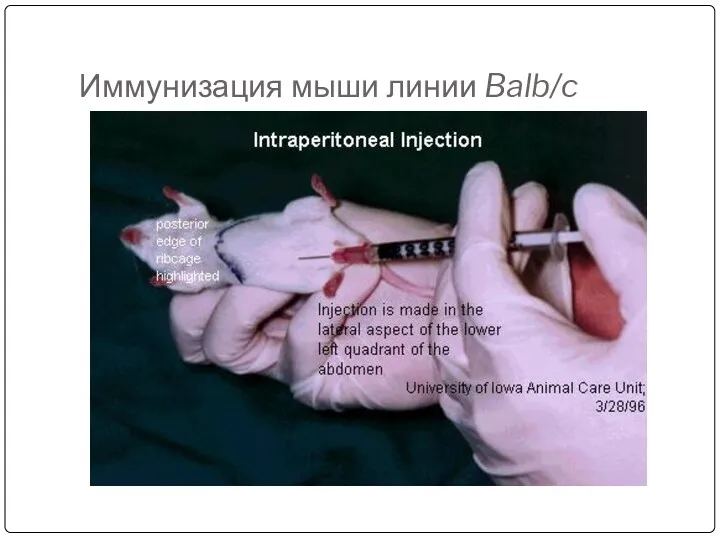
- 44. Immunize an animal usually mouse by injecting with an appropriate antigen along with Freund’s complete or
- 45. Иммунизация мыши линии Balb/c
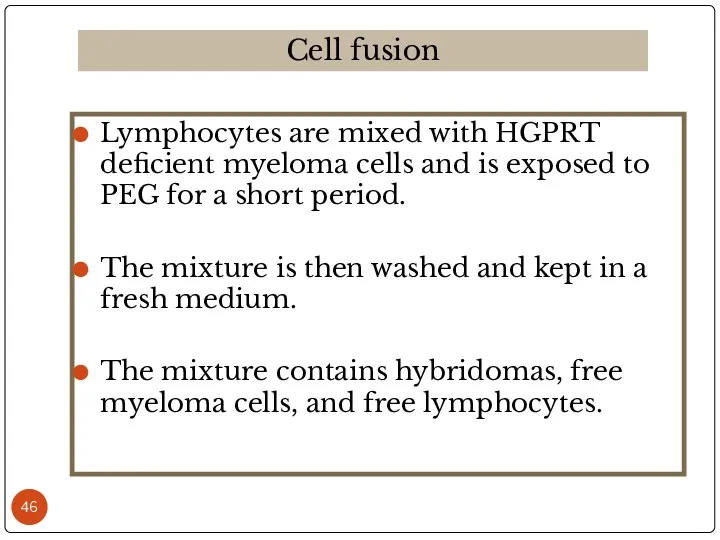
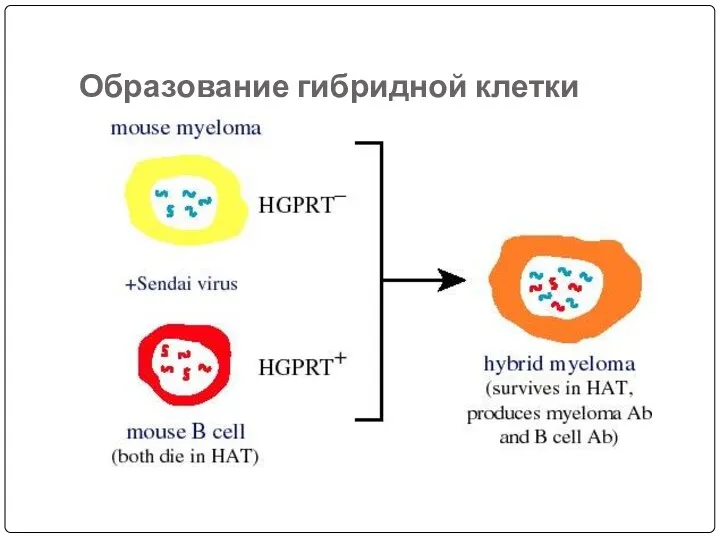
- 46. Lymphocytes are mixed with HGPRT deficient myeloma cells and is exposed to PEG for a short
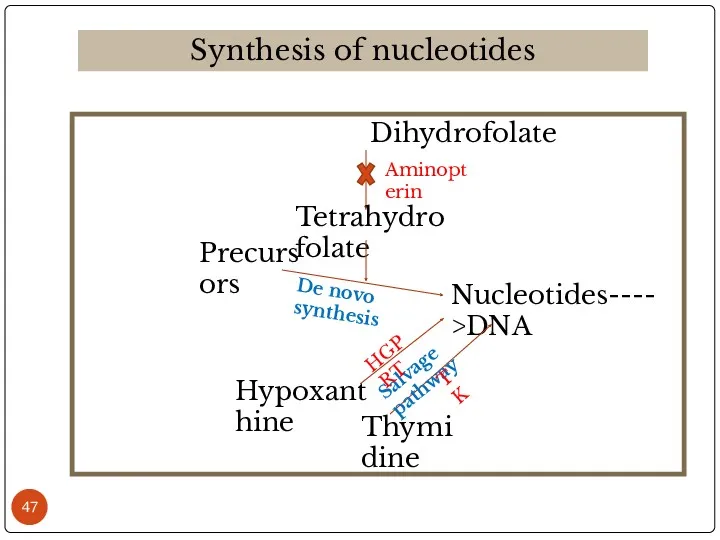
- 47. Dihydrofolate Synthesis of nucleotides Tetrahydrofolate Precursors Nucleotides---->DNA Hypoxanthine Thymidine De novo synthesis Salvage pathway Aminopterin HGPRT
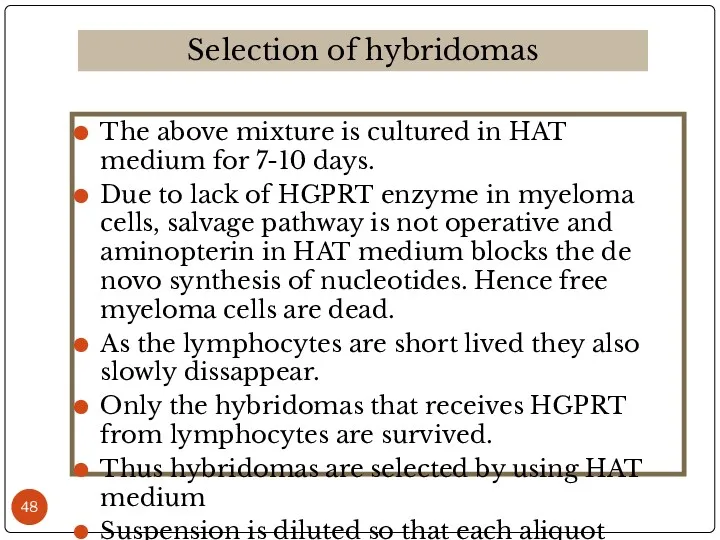
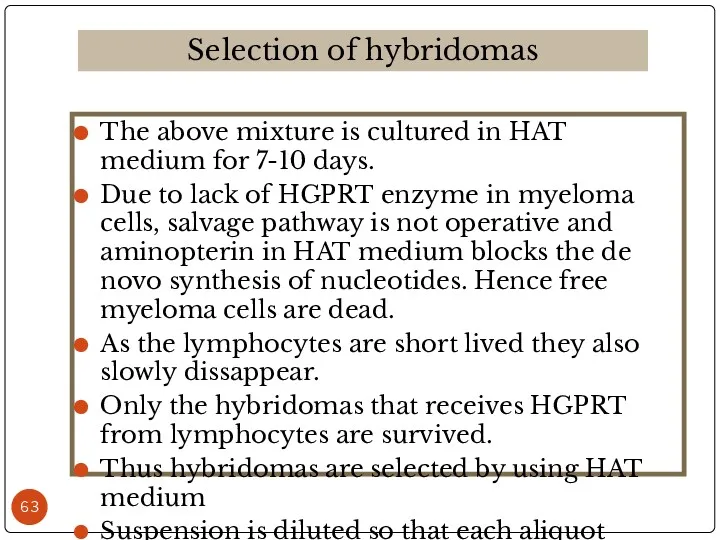
- 48. The above mixture is cultured in HAT medium for 7-10 days. Due to lack of HGPRT
- 49. Среда RPMI-1640
- 50. Сыворотка плода коровы
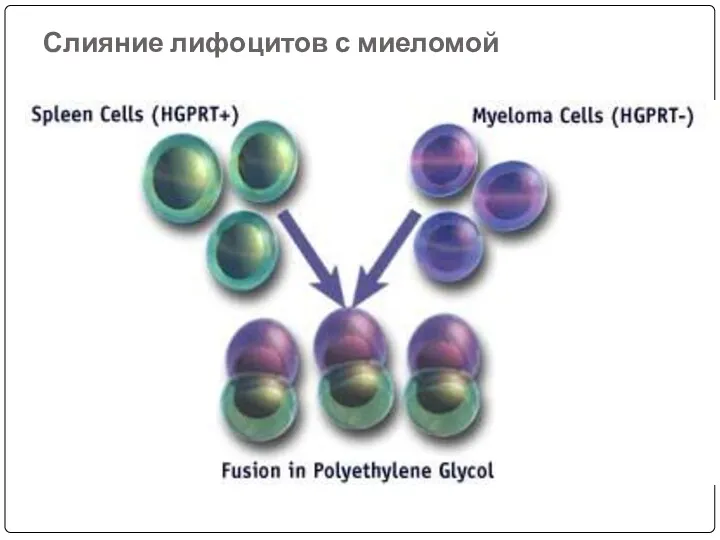
- 51. Слияние иммунных лимфоцитов с миеломой
- 52. 96-луночные планшеты для культуральных работ
- 53. Образование гибридной клетки
- 54. Слияние лифоцитов с миеломой
- 57. Распределение клеток по лункам планшеты
- 58. Культивирование гибридом в СО2 -инкубаторе
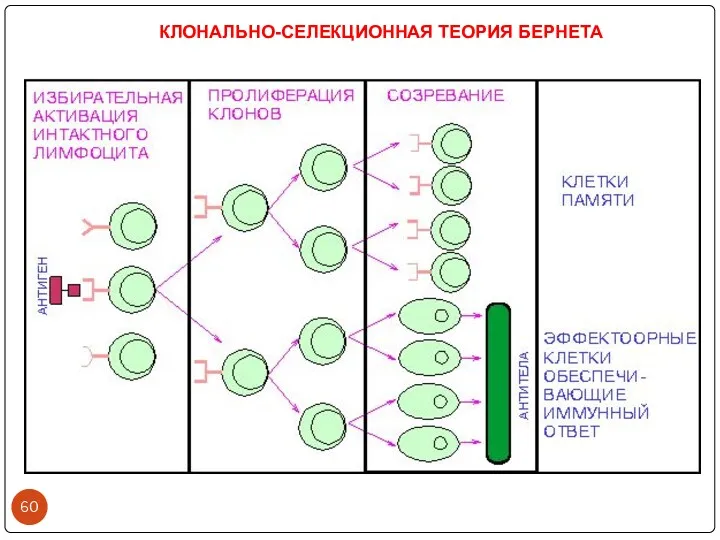
- 60. КЛОНАЛЬНО-СЕЛЕКЦИОННАЯ ТЕОРИЯ БЕРНЕТА
- 62. Виды клеток, образуемые в процессе слияния Неслившиеся клетки лимфоидного органа; Неслившиеся клетки миеломы; Гибриды лимфоцит+лимфоцит и
- 63. The above mixture is cultured in HAT medium for 7-10 days. Due to lack of HGPRT
- 64. Изоляция гибридов лимфоцит+миелома - от неслившихся лимфоцитов и гибридов лимфоцит+лимфоцит избавляться не нужно: через несколько дней
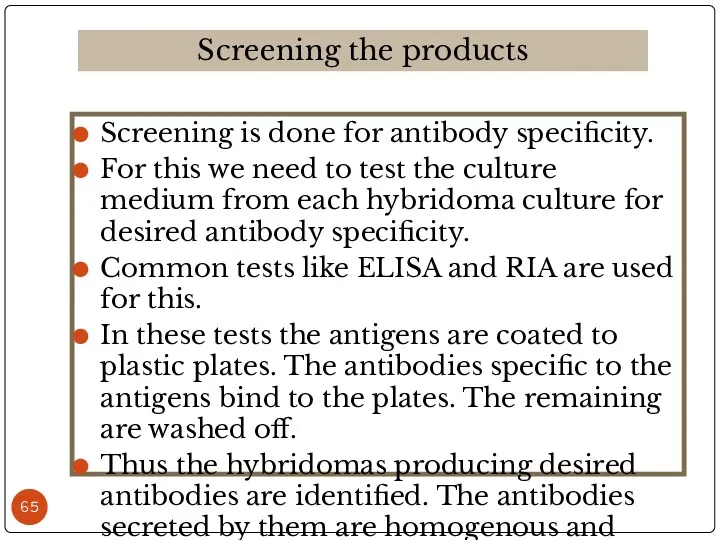
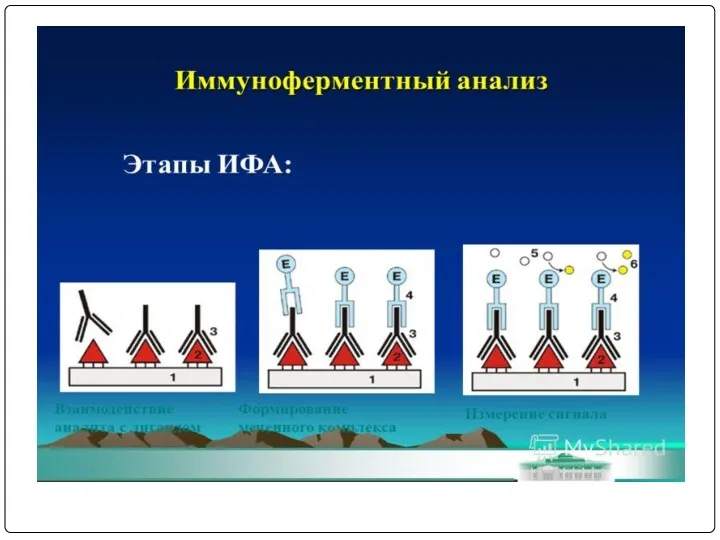
- 65. Screening is done for antibody specificity. For this we need to test the culture medium from
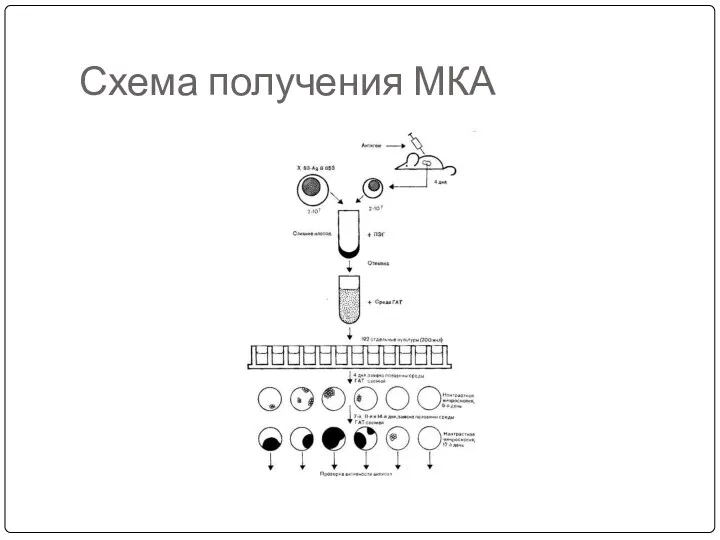
- 67. Схема получения МКА
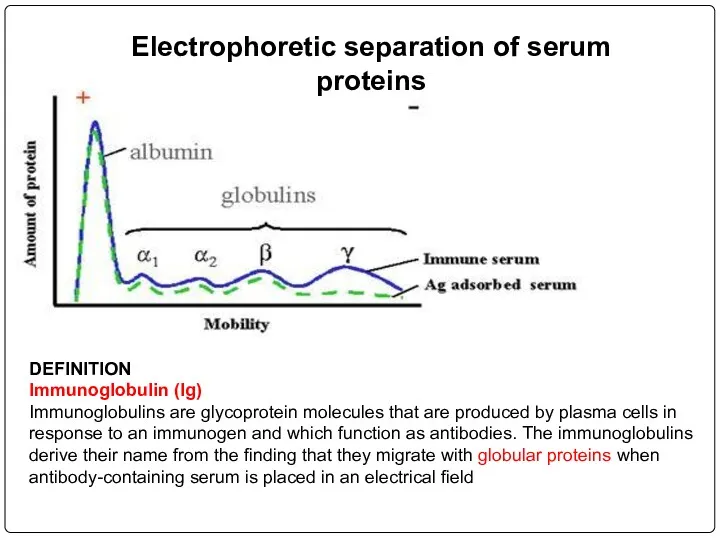
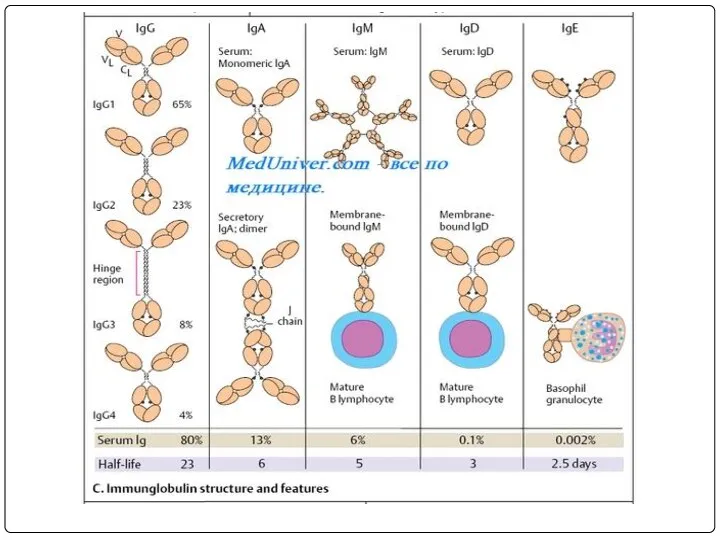
- 68. Electrophoretic separation of serum proteins DEFINITION Immunoglobulin (Ig) Immunoglobulins are glycoprotein molecules that are produced by
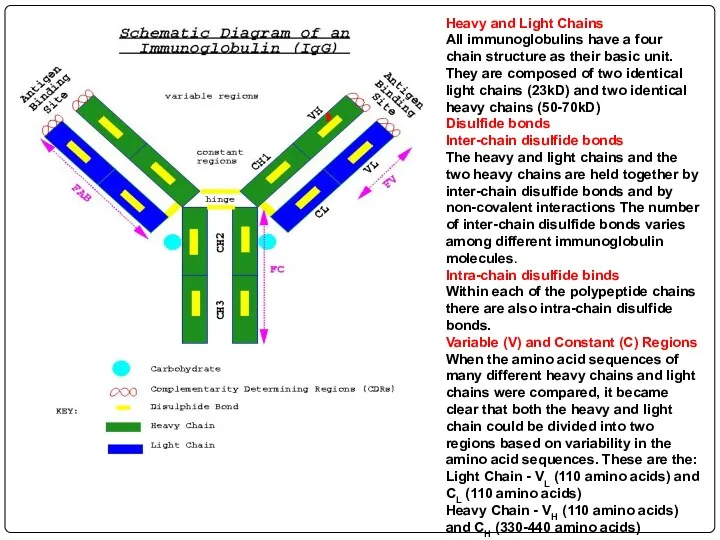
- 70. Heavy and Light Chains All immunoglobulins have a four chain structure as their basic unit. They
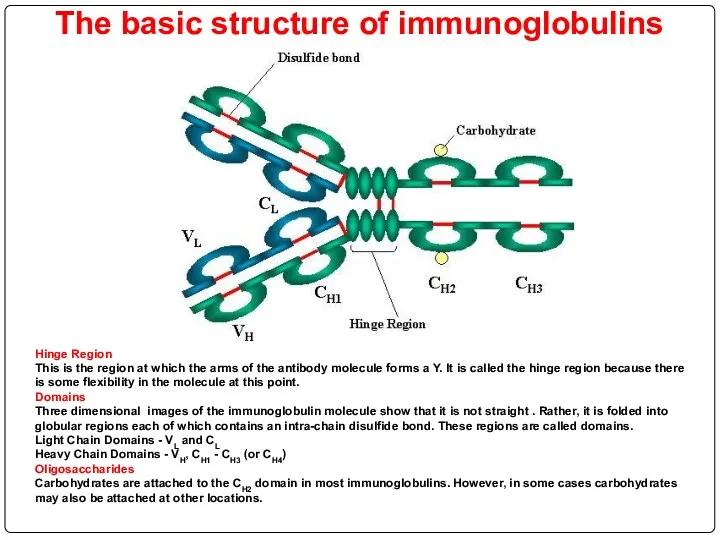
- 71. The basic structure of immunoglobulins Hinge Region This is the region at which the arms of
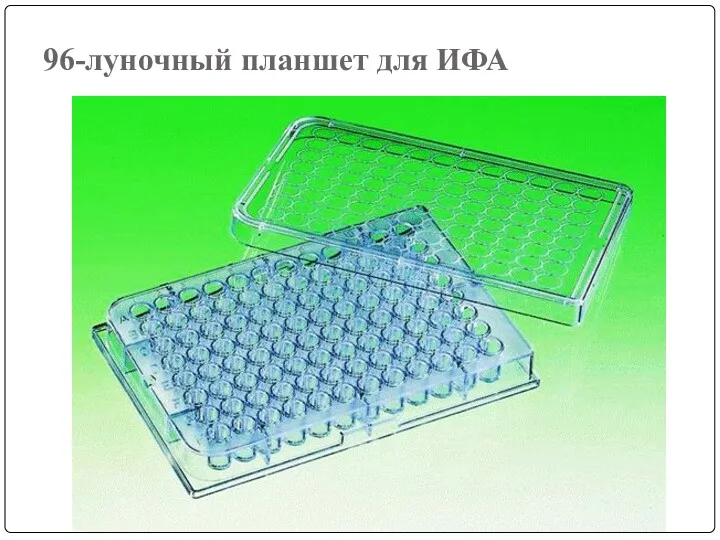
- 75. 96-луночный планшет для ИФА
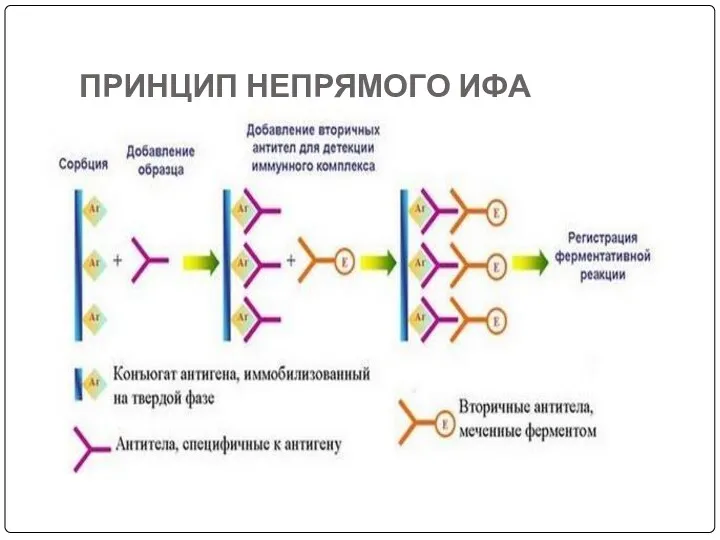
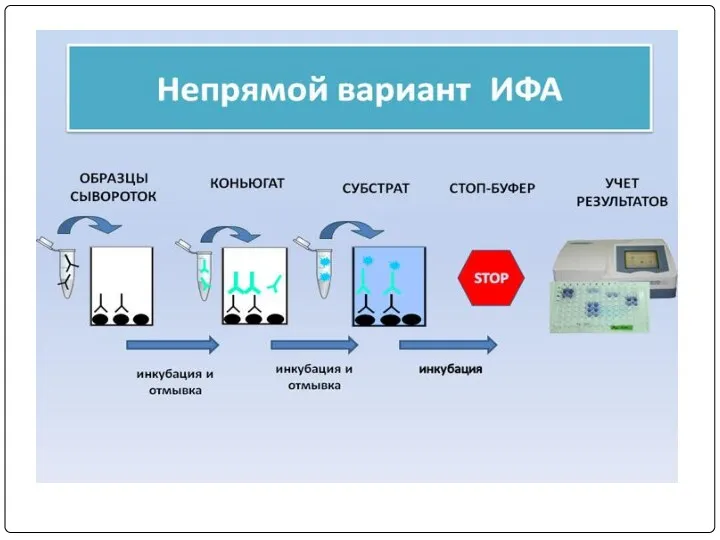
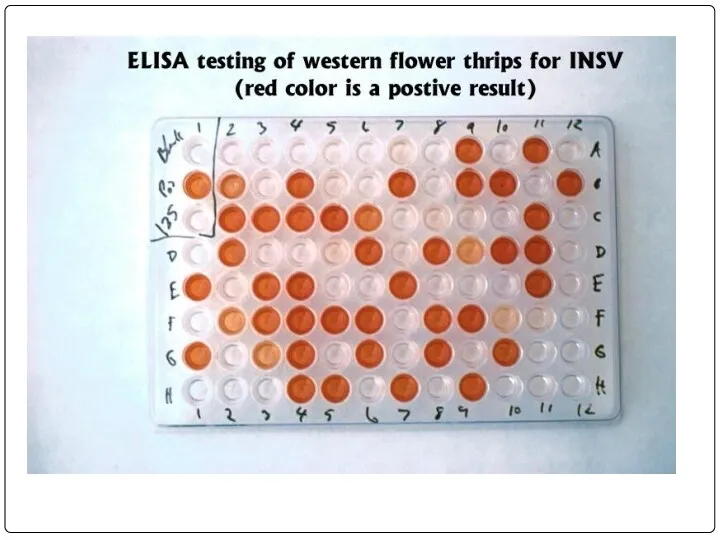
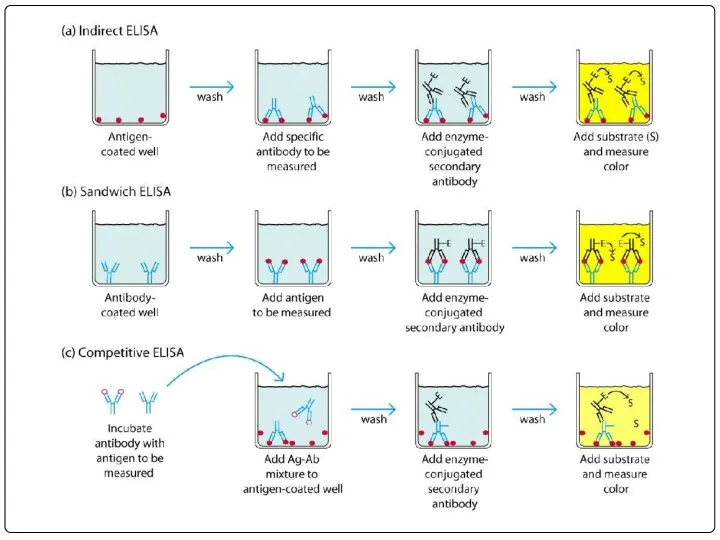
- 76. ПРИНЦИП НЕПРЯМОГО ИФА
- 80. Спектрофотометр для ИФА
- 81. The single hybrid cell producing the desired antibody are isolated and cloned. Usually two techniques are
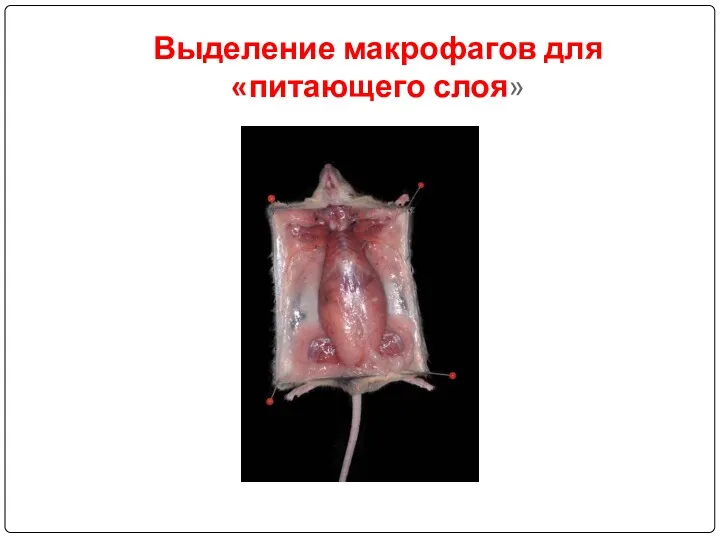
- 82. Выделение макрофагов для «питающего слоя»
- 83. 96-луночные планшеты для культуральных работ
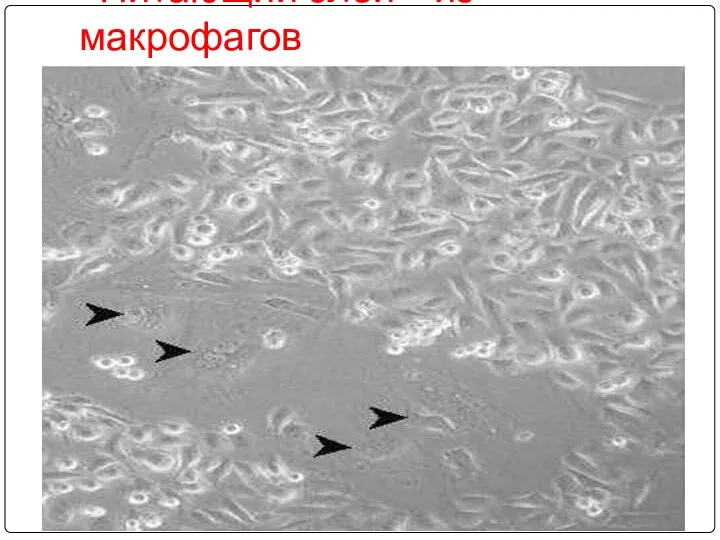
- 84. «Питающий слой» из макрофагов
- 85. Biochemical and biophysical characterization are made for desired specificity. It is important to note the monoclonal
- 86. Хранение клеток в жидком азоте
- 87. Разморозка гибридомных клеток
- 88. Накопление МКА в матрасах
- 89. Наработка МКА в асцитной жидкости
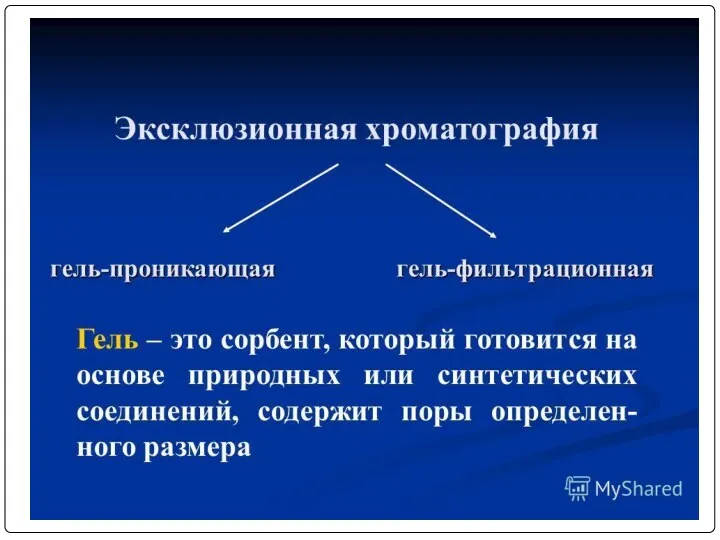
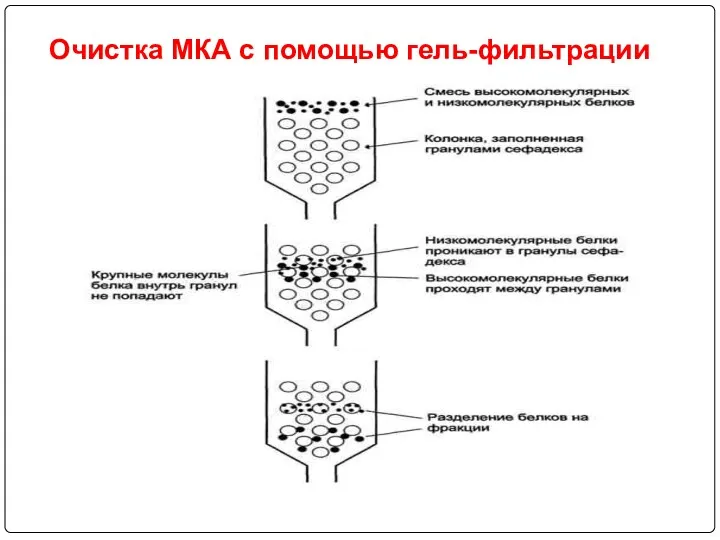
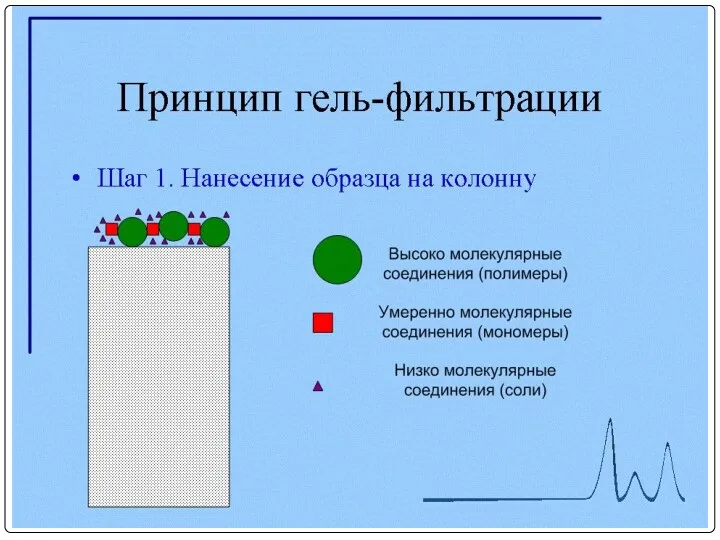
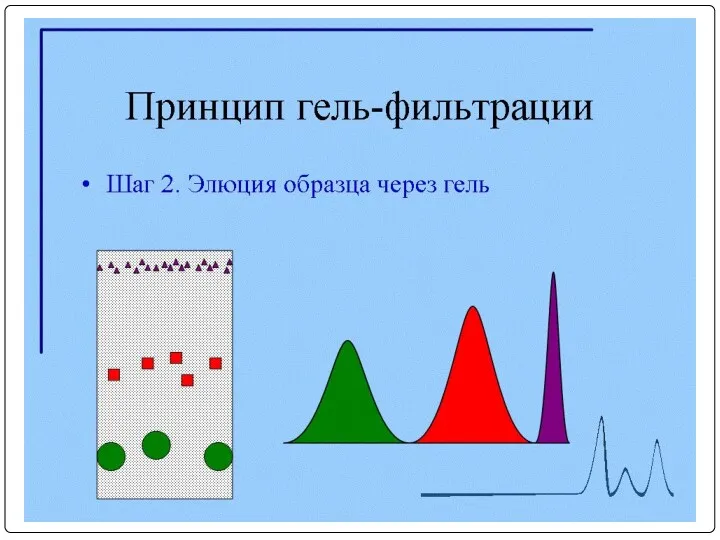
- 92. Очистка МКА с помощью гель-фильтрации
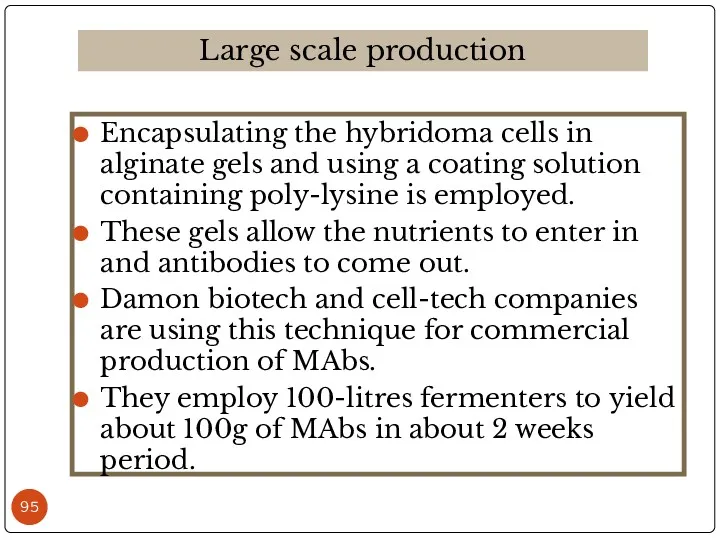
- 95. Encapsulating the hybridoma cells in alginate gels and using a coating solution containing poly-lysine is employed.
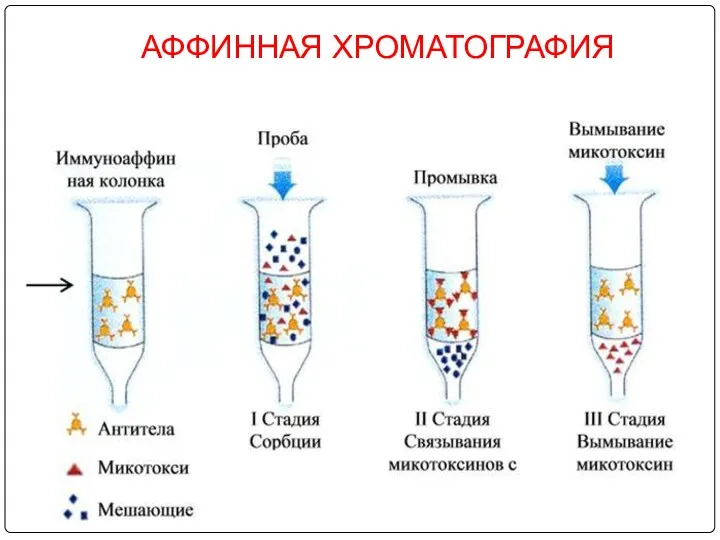
- 96. АФФИННАЯ ХРОМАТОГРАФИЯ
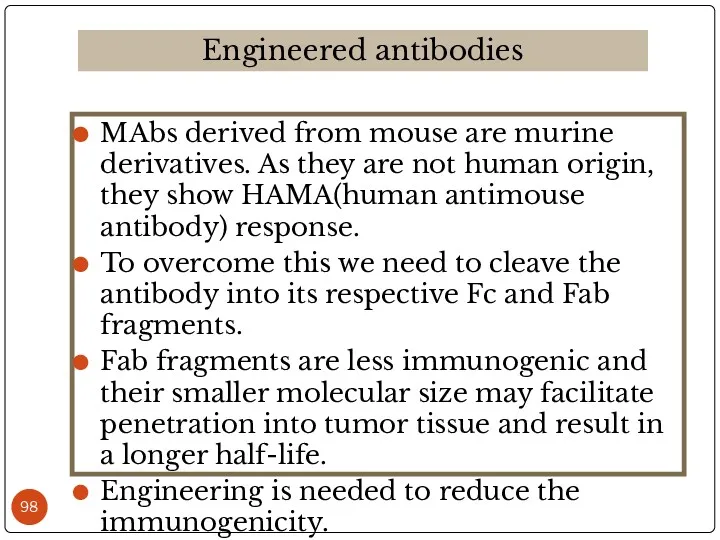
- 98. MAbs derived from mouse are murine derivatives. As they are not human origin, they show HAMA(human
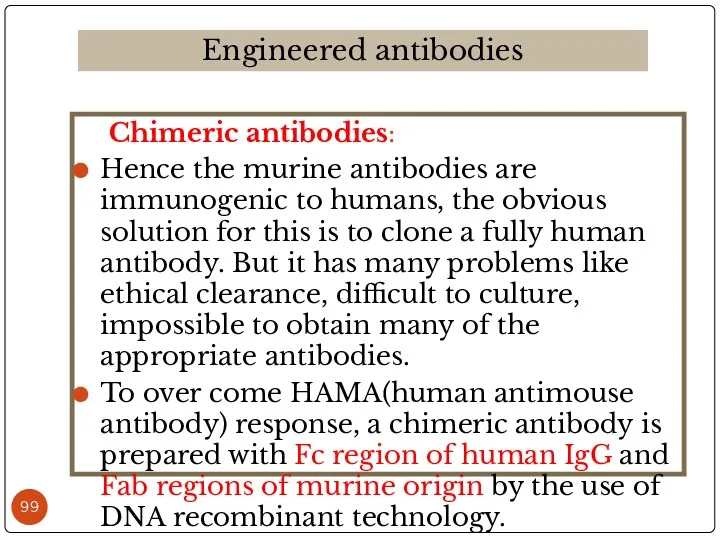
- 99. Chimeric antibodies: Hence the murine antibodies are immunogenic to humans, the obvious solution for this is
- 100. Основные проблемы, возникающие при использовании монАТ в терапии а) Подавляющее большинство получаемых монАТ имеет животное происхождение
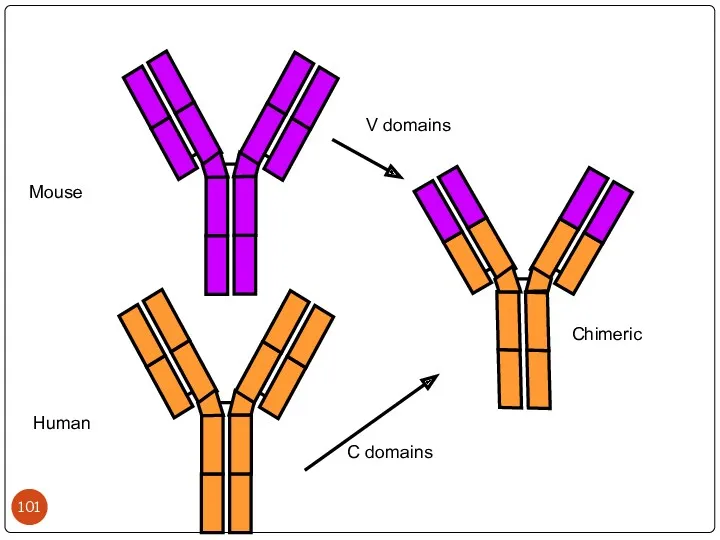
- 101. Mouse Human Chimeric V domains C domains
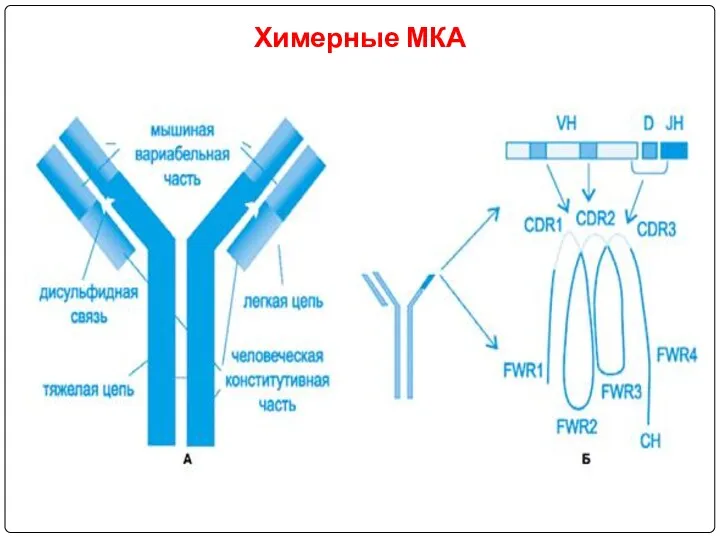
- 102. Химерные МКА
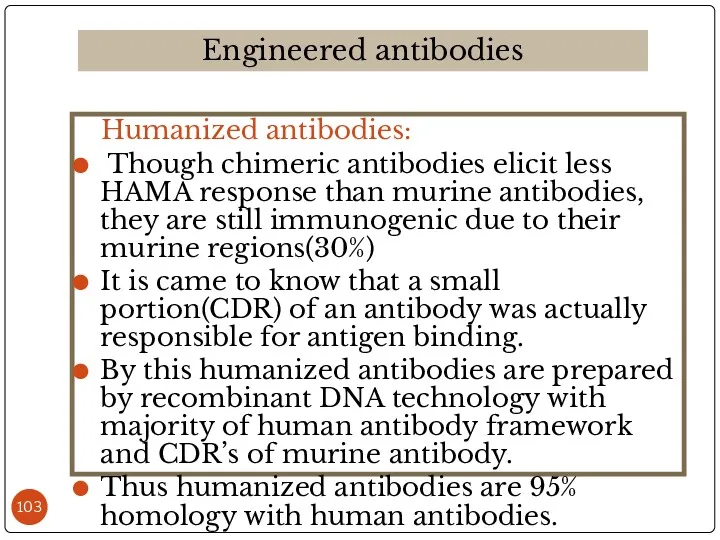
- 103. Humanized antibodies: Though chimeric antibodies elicit less HAMA response than murine antibodies, they are still immunogenic
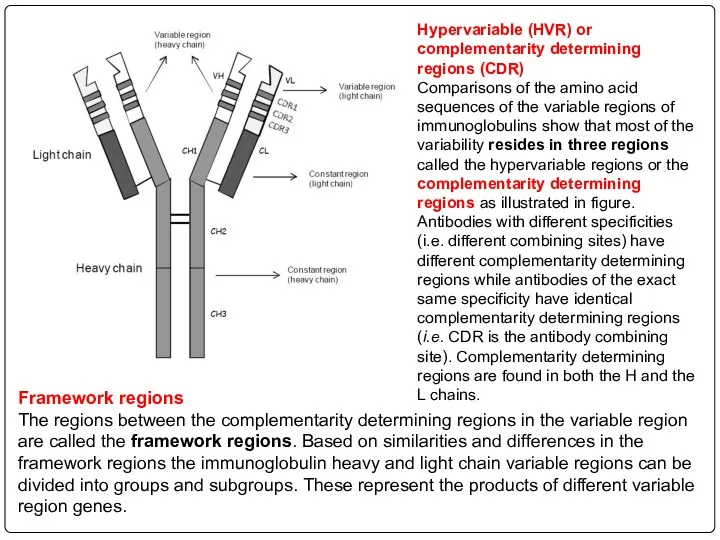
- 104. Hypervariable (HVR) or complementarity determining regions (CDR) Comparisons of the amino acid sequences of the variable
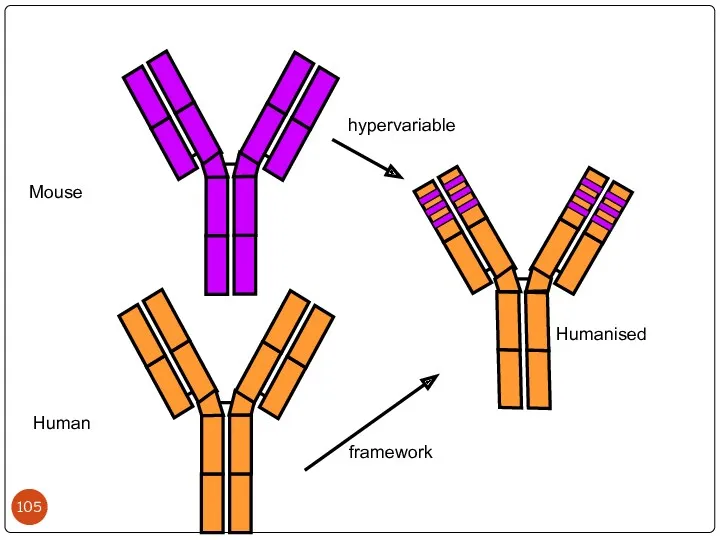
- 105. Mouse Human Humanised hypervariable framework
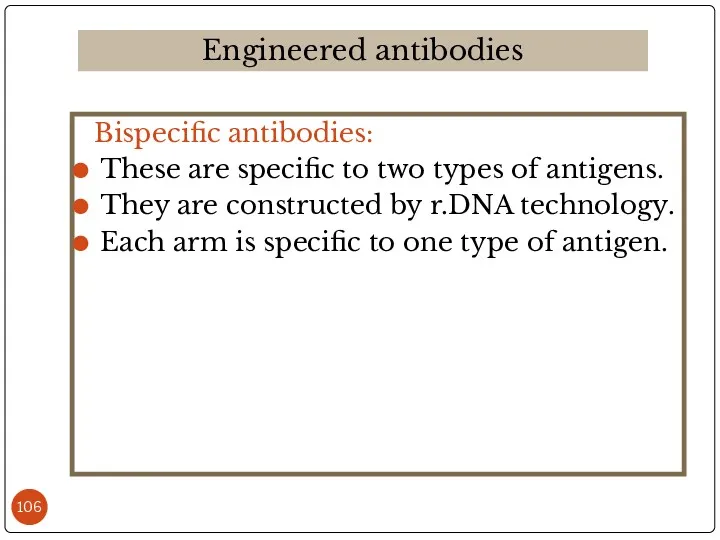
- 106. Bispecific antibodies: These are specific to two types of antigens. They are constructed by r.DNA technology.
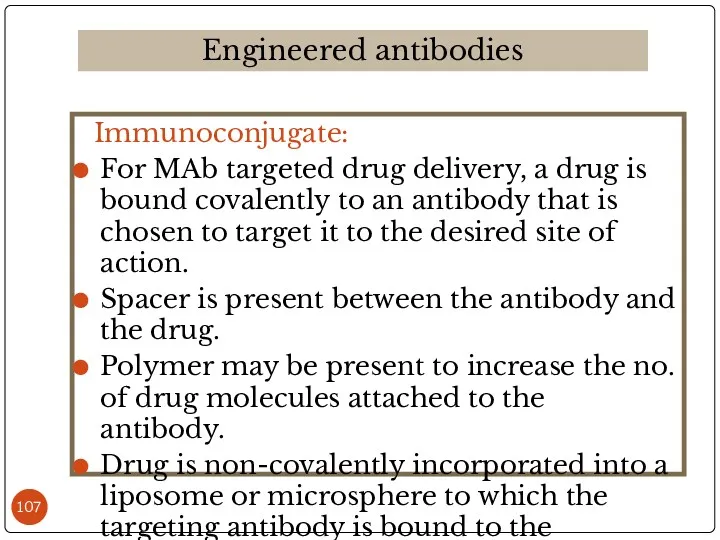
- 107. Immunoconjugate: For MAb targeted drug delivery, a drug is bound covalently to an antibody that is
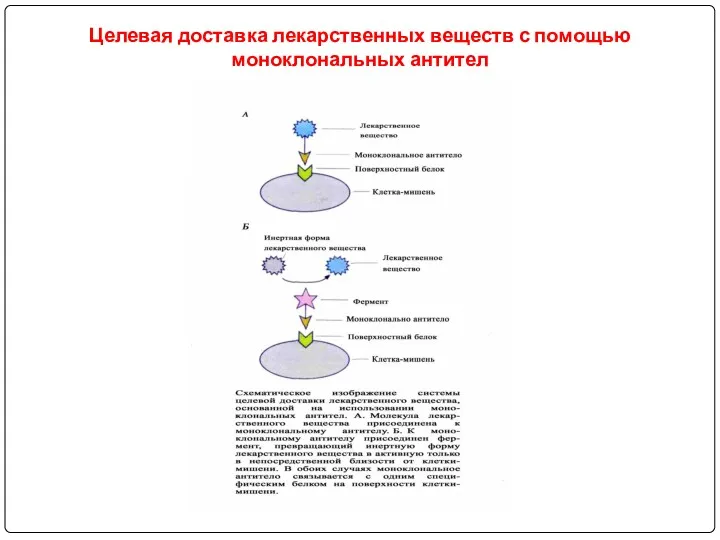
- 108. Целевая доставка лекарственных веществ с помощью моноклональных антител
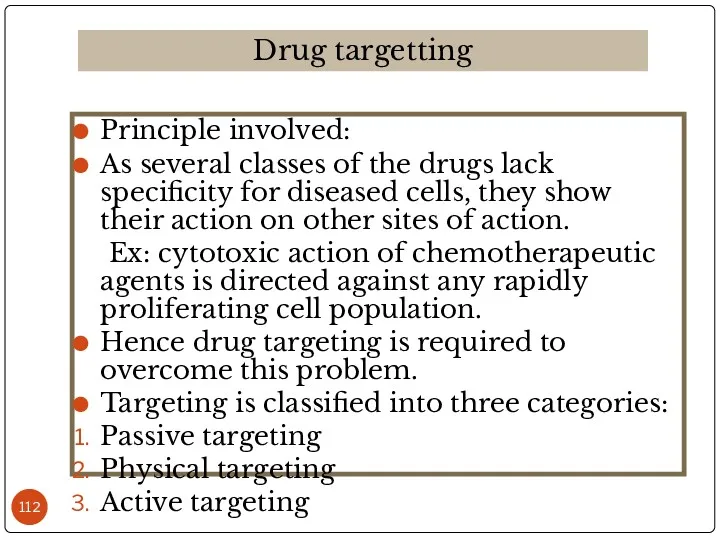
- 112. Principle involved: As several classes of the drugs lack specificity for diseased cells, they show their
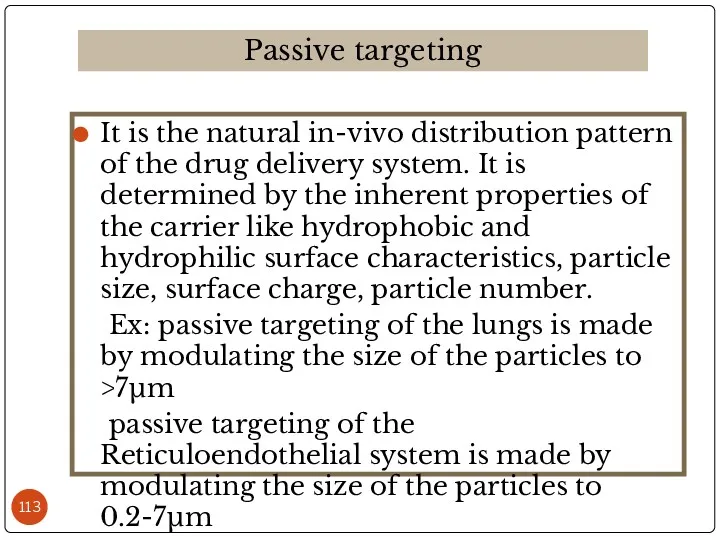
- 113. It is the natural in-vivo distribution pattern of the drug delivery system. It is determined by
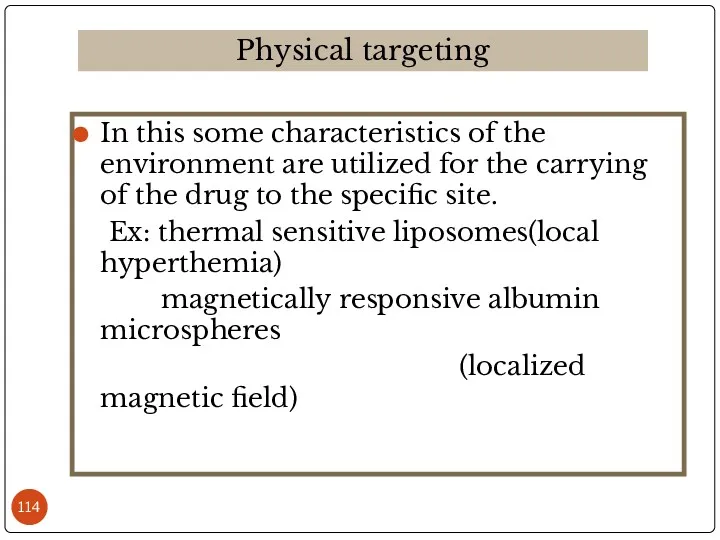
- 114. In this some characteristics of the environment are utilized for the carrying of the drug to
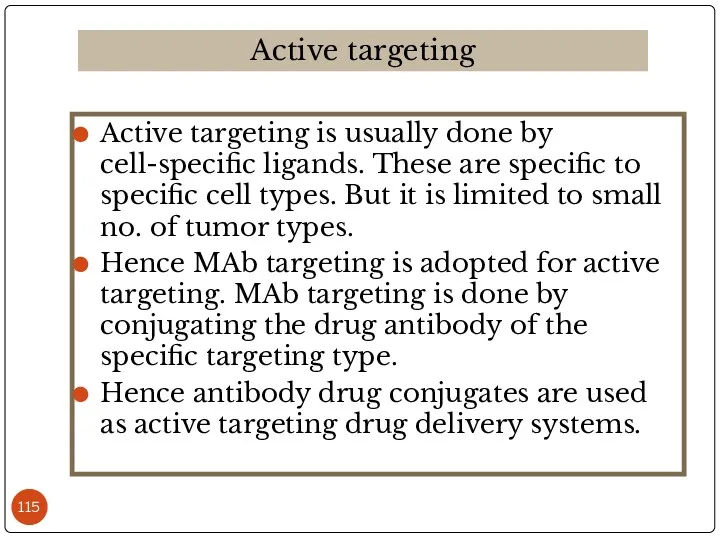
- 115. Active targeting is usually done by cell-specific ligands. These are specific to specific cell types. But
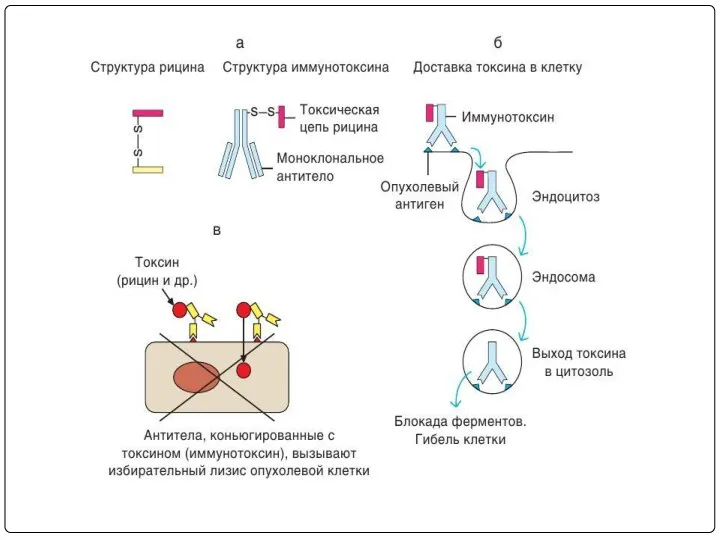
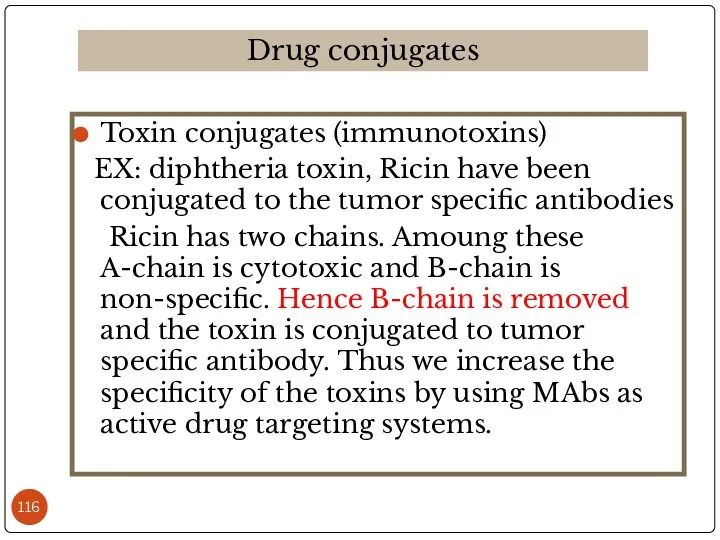
- 116. Toxin conjugates (immunotoxins) EX: diphtheria toxin, Ricin have been conjugated to the tumor specific antibodies Ricin
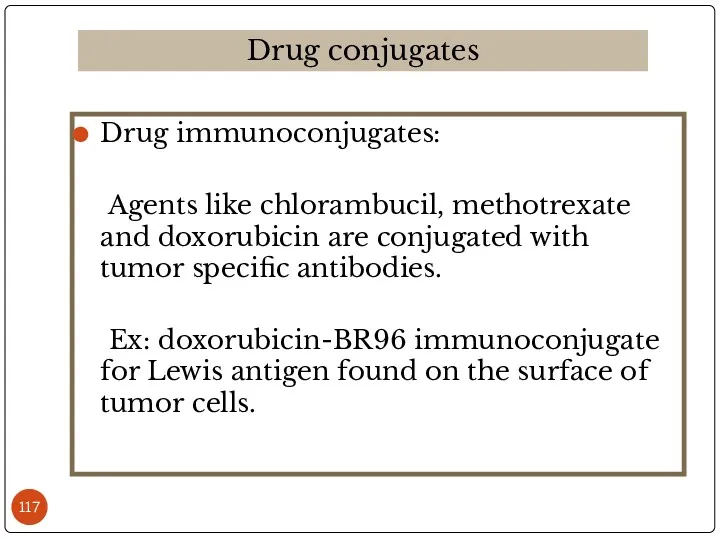
- 117. Drug immunoconjugates: Agents like chlorambucil, methotrexate and doxorubicin are conjugated with tumor specific antibodies. Ex: doxorubicin-BR96
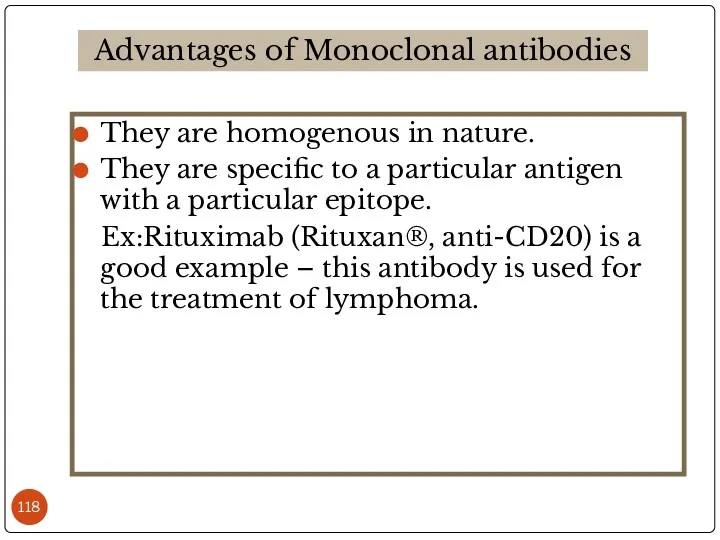
- 118. They are homogenous in nature. They are specific to a particular antigen with a particular epitope.
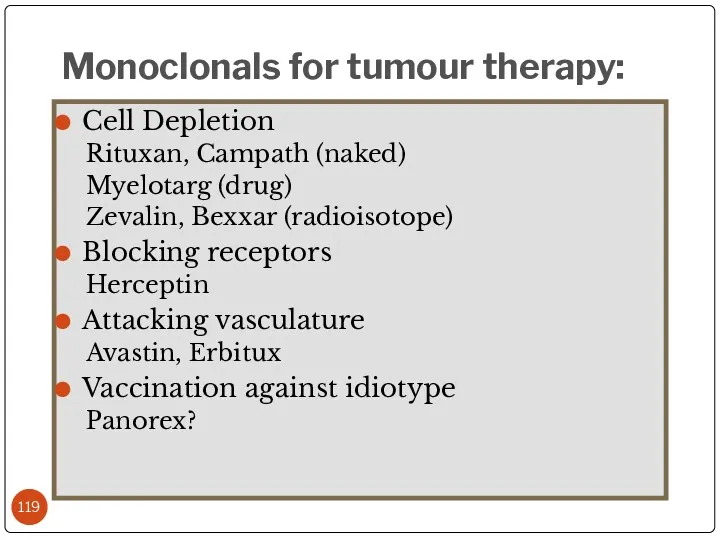
- 119. Cell Depletion Rituxan, Campath (naked) Myelotarg (drug) Zevalin, Bexxar (radioisotope) Blocking receptors Herceptin Attacking vasculature Avastin,
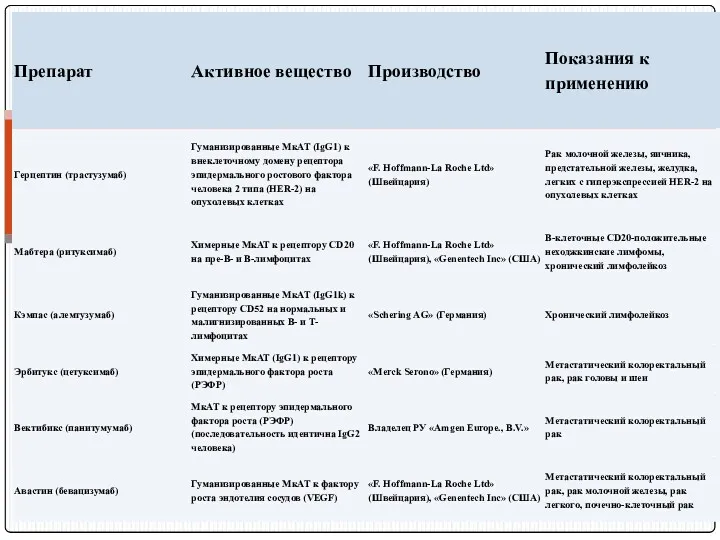
- 120. Препараты МкАТ, используемые при лечении онкологических болезнях
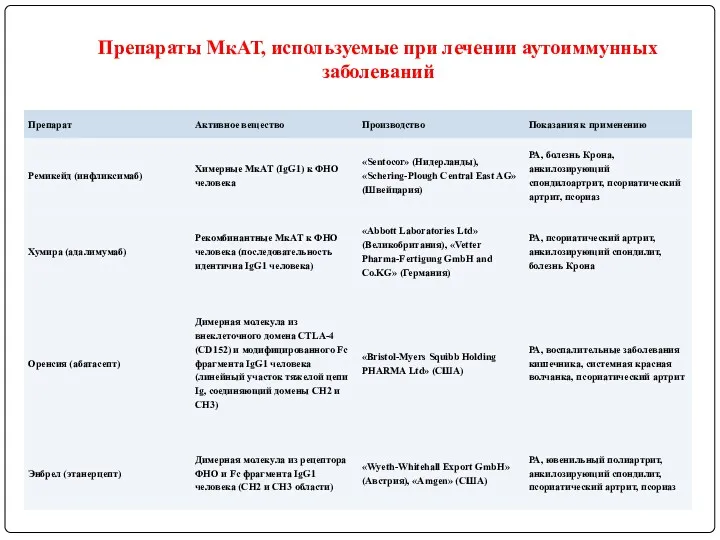
- 121. Препараты МкАТ, используемые при лечении аутоиммунных заболеваний
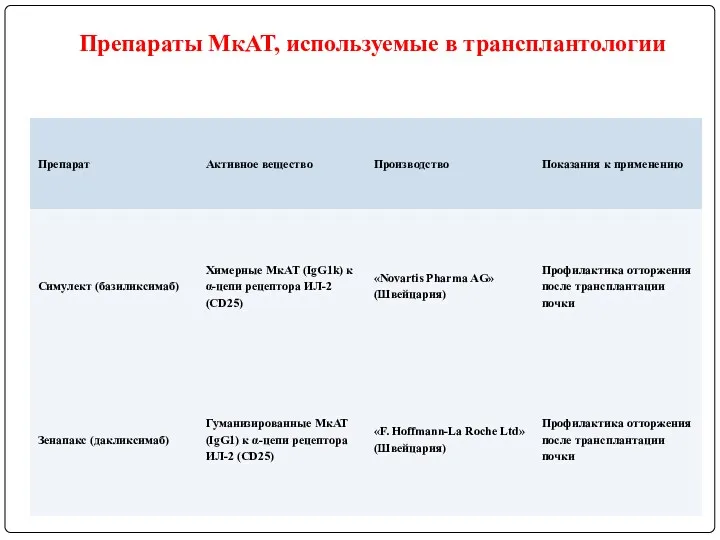
- 122. Препараты МкАТ, используемые в трансплантологии
- 124. Скачать презентацию

























 Первая помощь ожогах
Первая помощь ожогах Характеристики основных видов твердых лекарственных форм
Характеристики основных видов твердых лекарственных форм Биоревитализация. Базовая процедура в эстетической медицине
Биоревитализация. Базовая процедура в эстетической медицине Malaria
Malaria Значение физических упражнений для формирования системы опоры и движения
Значение физических упражнений для формирования системы опоры и движения Иммунопатологические состояния
Иммунопатологические состояния Диагностика, лечение и профилактика заболеваний, сопровождающихся легочной гипертензией, во всех возрастных группах
Диагностика, лечение и профилактика заболеваний, сопровождающихся легочной гипертензией, во всех возрастных группах Өлім. Өлім белгілері. Танатогенез. Терминальді жағдай, өлімнен кейінгі өзгерістер
Өлім. Өлім белгілері. Танатогенез. Терминальді жағдай, өлімнен кейінгі өзгерістер Гигиенические требования к хирургическим отделениям больниц
Гигиенические требования к хирургическим отделениям больниц Современные ингаляционные анестетики
Современные ингаляционные анестетики Группы крови. Переливание крови
Группы крови. Переливание крови Патология внешнего дыхания
Патология внешнего дыхания Лекарственные препараты
Лекарственные препараты Внутрибольничные инфекции
Внутрибольничные инфекции Неотложные состояния в акушерстве и гинекологии на догоспитальном этапе
Неотложные состояния в акушерстве и гинекологии на догоспитальном этапе Диетология. Влияние витамина В1 на аппетит
Диетология. Влияние витамина В1 на аппетит Предстерилизационная очистка изделий медицинского назначения
Предстерилизационная очистка изделий медицинского назначения Ультразвуковая диагностика при подозрении на опухоль щитовидной железы
Ультразвуковая диагностика при подозрении на опухоль щитовидной железы Неонатальный период. Доношенный новорожденный. Тема 3
Неонатальный период. Доношенный новорожденный. Тема 3 Сестринское обследование - первый этап сестринского процесса
Сестринское обследование - первый этап сестринского процесса Тісжегінің таралуы мен белсенділігін бағалау. Пародонт тіндерінің зақымдалу белсенділігі мен таралуын бағалау
Тісжегінің таралуы мен белсенділігін бағалау. Пародонт тіндерінің зақымдалу белсенділігі мен таралуын бағалау Сифилис, туберкулез
Сифилис, туберкулез Патофизиология эндокринной системы
Патофизиология эндокринной системы Синдром диссеминированного внутрисосудистого свертывания крови (ДВС-синдром) у беременных
Синдром диссеминированного внутрисосудистого свертывания крови (ДВС-синдром) у беременных Лечебная физическая культура при травмах и заболеваниях центральной нервной системы
Лечебная физическая культура при травмах и заболеваниях центральной нервной системы Контроль качества в здравоохранении
Контроль качества в здравоохранении Туляремия дами
Туляремия дами Острая печеночная недостаточность
Острая печеночная недостаточность