Содержание
- 2. Right Heart Catheterization Swan-Ganz Catheter: History Jeremy Swan (1922-2005), an Irish cardiologist, worked in the Mayo
- 3. Swan-Ganz Catheter: History Jeremy Swan (1922-2005), an Irish cardiologist, worked in the Mayo Clinic, Rochester, and
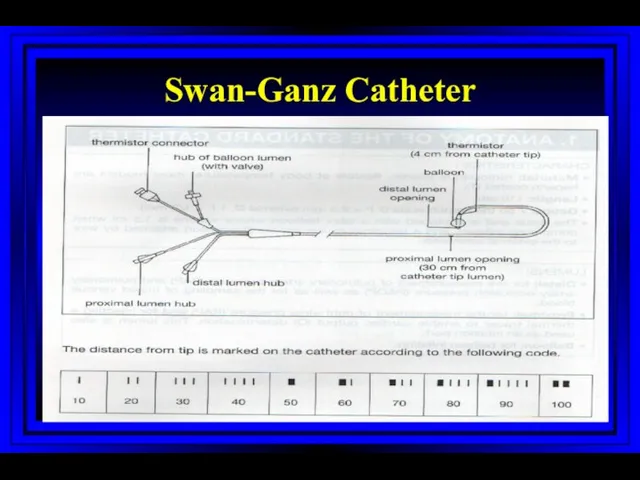
- 4. Swan-Ganz Catheter
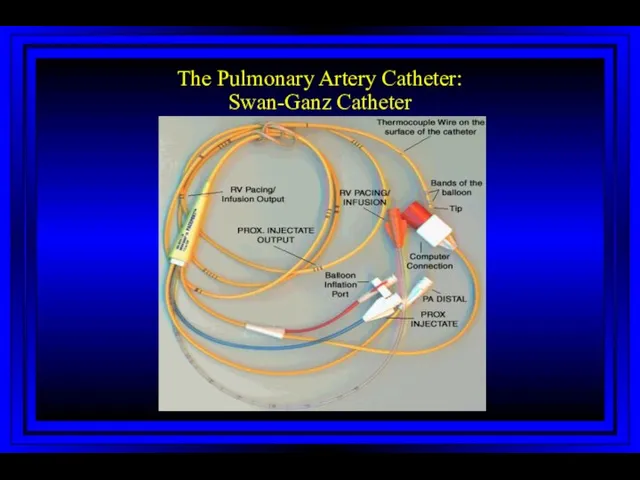
- 5. The Pulmonary Artery Catheter: Swan-Ganz Catheter
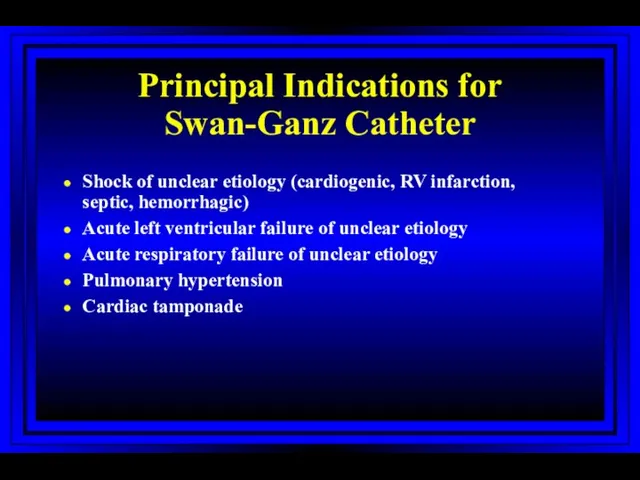
- 6. Principal Indications for Swan-Ganz Catheter Shock of unclear etiology (cardiogenic, RV infarction, septic, hemorrhagic) Acute left
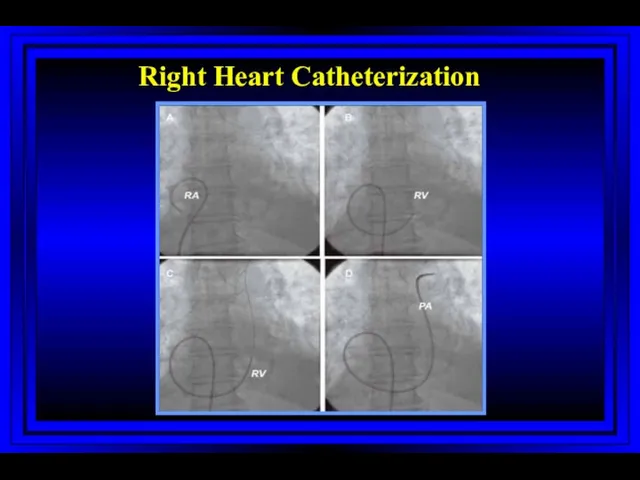
- 7. Right Heart Catheterization
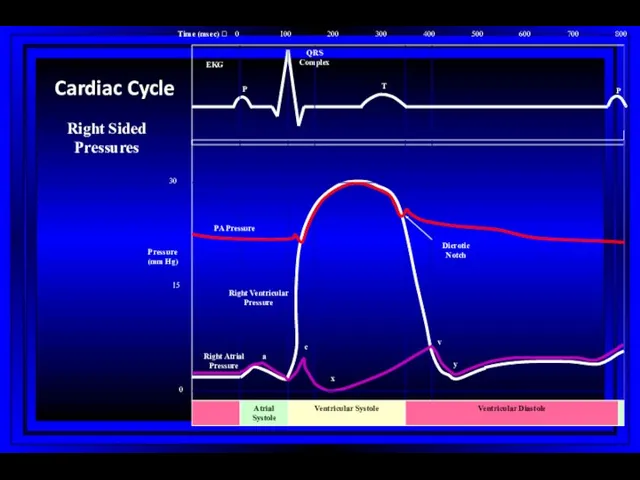
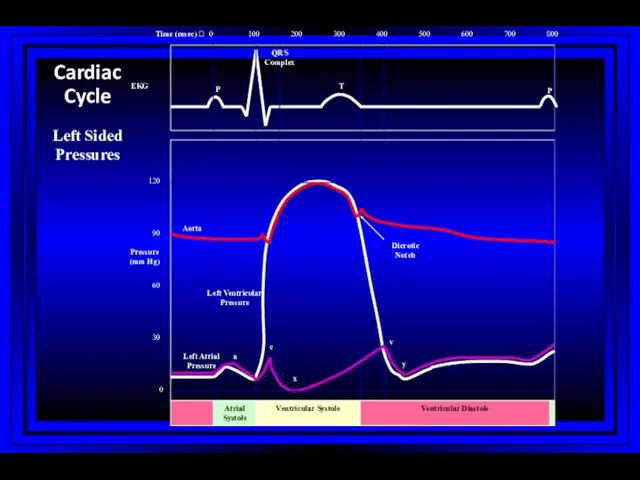
- 8. 0 100 200 300 400 500 600 700 800 0 15 30 Atrial Systole Ventricular Systole
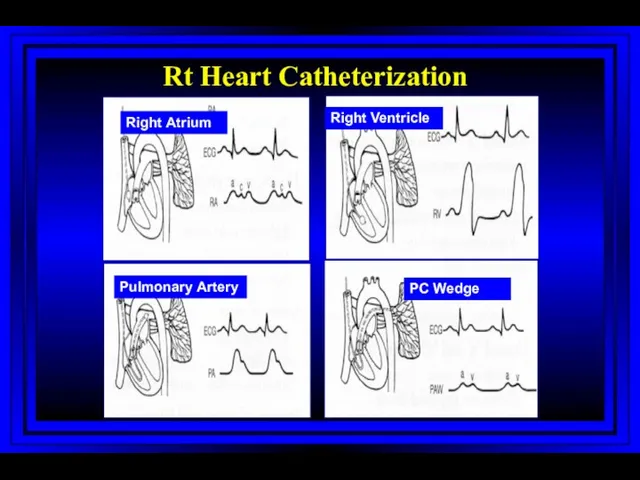
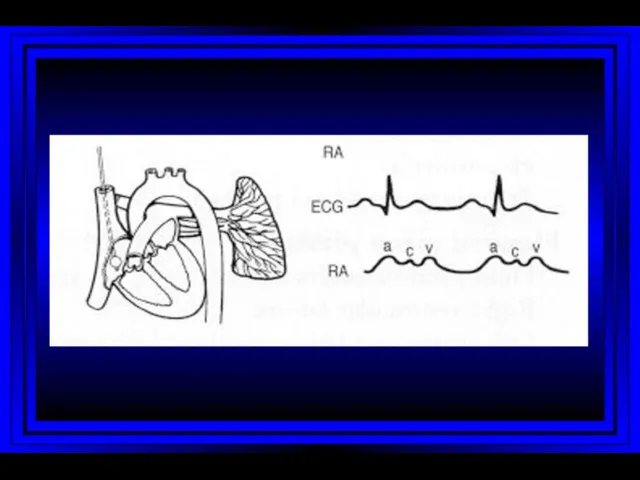
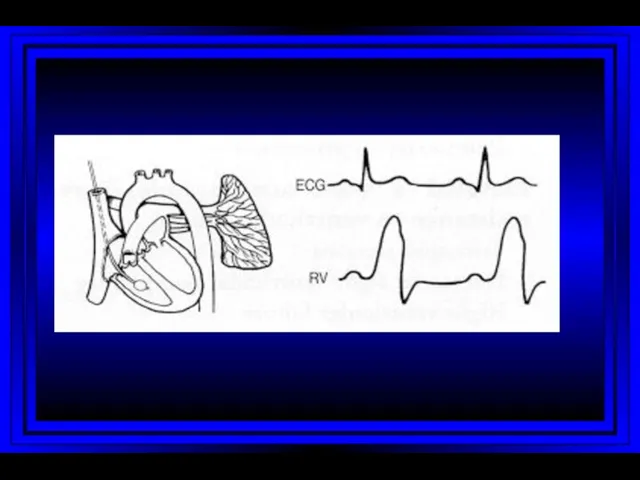
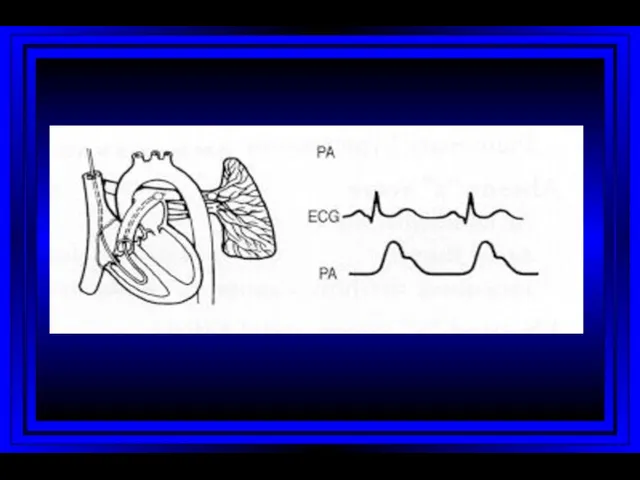
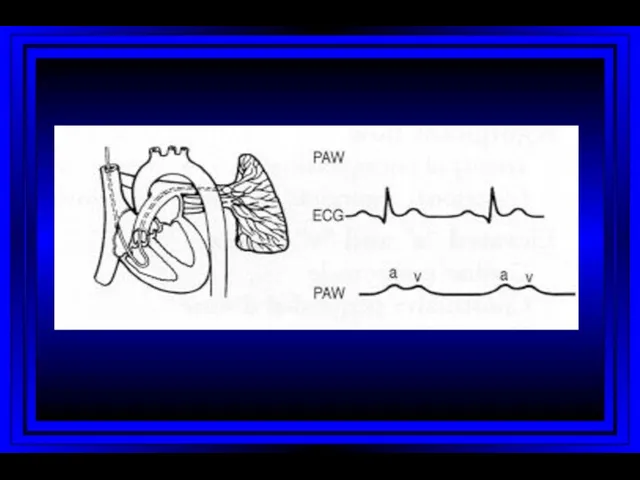
- 9. Right Atrium Right Ventricle Pulmonary Artery PC Wedge Rt Heart Catheterization
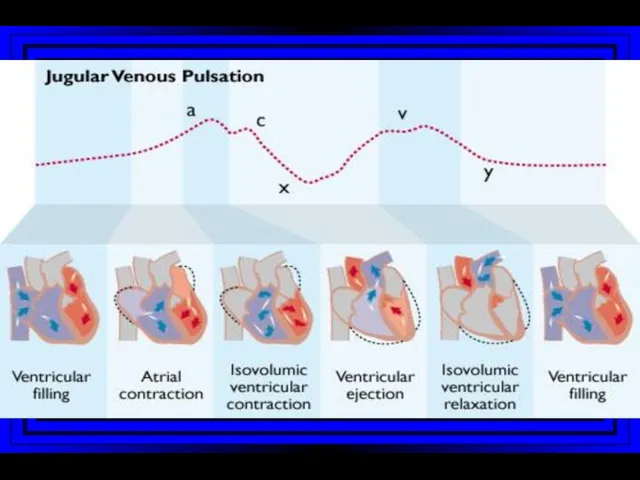
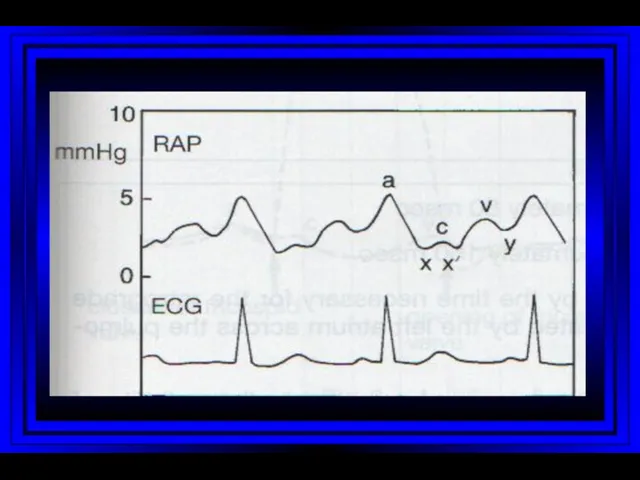
- 10. Jugular Venous Pulsations A wave – backward flow of blood produced after atrial contraction C wave
- 16. 0 100 200 300 400 500 600 700 800 0 30 60 90 120 Atrial Systole
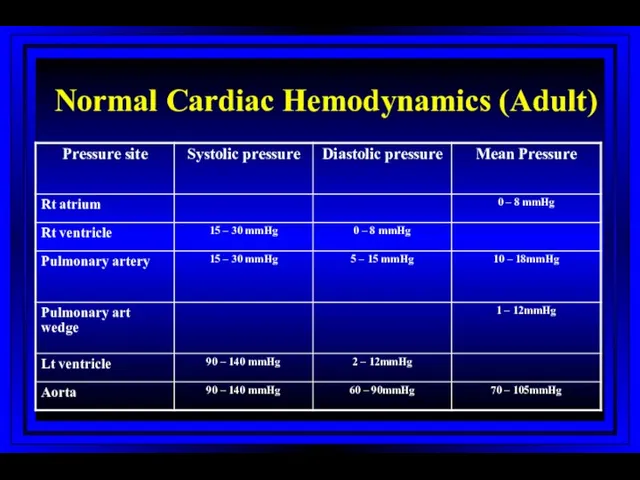
- 18. Normal Cardiac Hemodynamics (Adult)
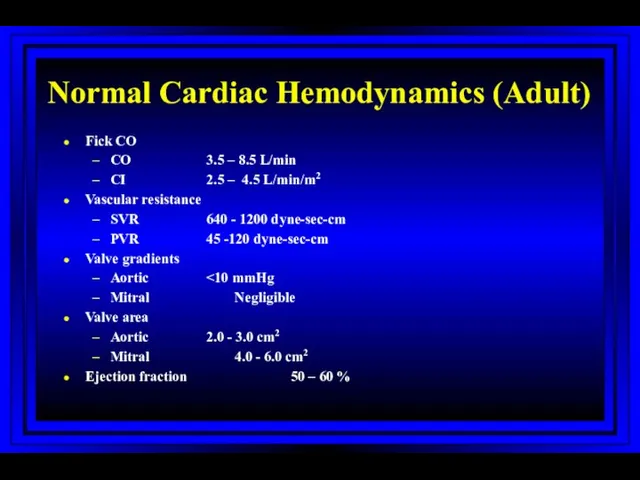
- 19. Normal Cardiac Hemodynamics (Adult) Fick CO CO 3.5 – 8.5 L/min CI 2.5 – 4.5 L/min/m2
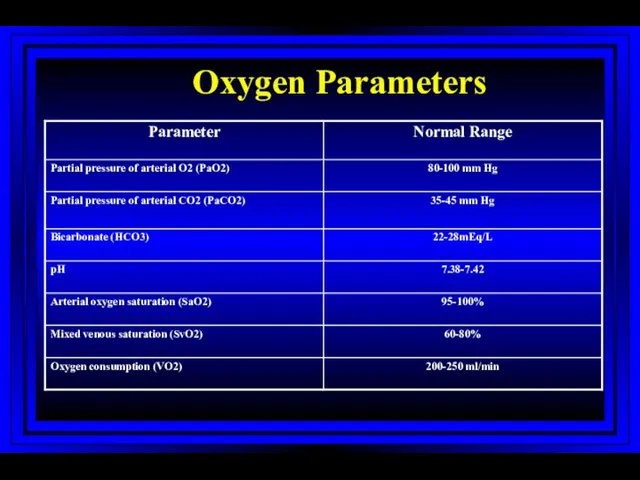
- 20. Oxygen Parameters
- 21. Normal Pressures LA and PCW: Mean 4-12mmHg Aorta: Systolic 90-140mmHg Diastolic 60-90mmHg Mean 70-105mmHg Left Ventricle:
- 22. Measured Variables Mean and phasic arterial blood pressure Heart rate Mean right atrial pressure/waves Systolic and
- 23. Calculated Variables Cardiac index Stroke index Systemic vascular resistance Pulmonary vascular resistance Shunts Ventricular function Valvular
- 24. Stenotic Orifices Gradients Valve orifice cross-sectional areas Measurements assist in making decisions regarding surgical intervention
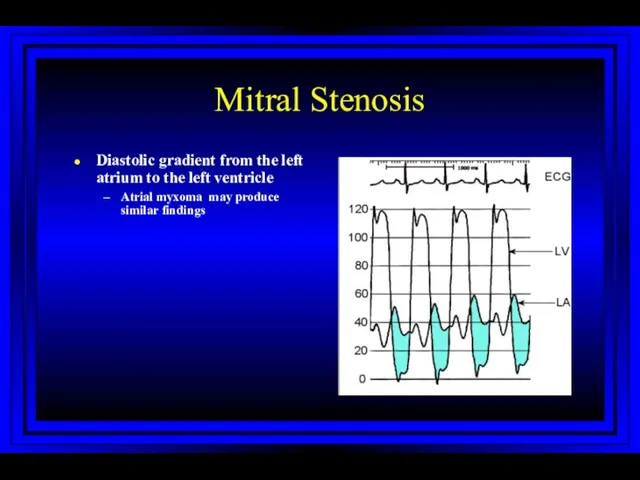
- 26. Mitral Stenosis Diastolic gradient from the left atrium to the left ventricle Atrial myxoma may produce
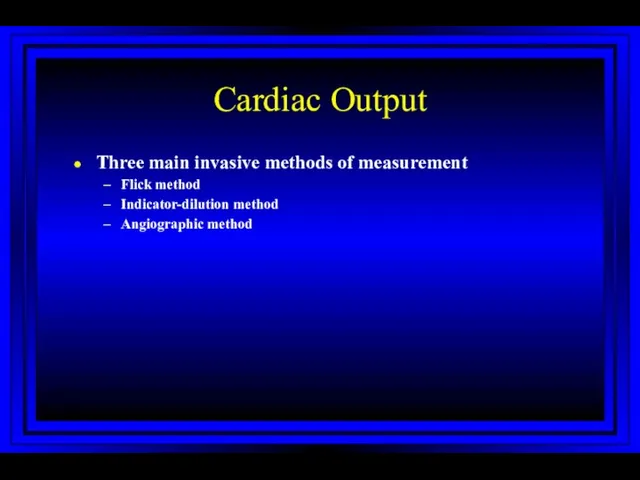
- 27. Cardiac Output Three main invasive methods of measurement Flick method Indicator-dilution method Angiographic method
- 28. Fick Method The amount of oxygen extracted by the lungs from air = The amount taken
- 29. The Indicator-dilution Technique and Thermodilution Technique Dilution of an indicator is proportional to the volume of
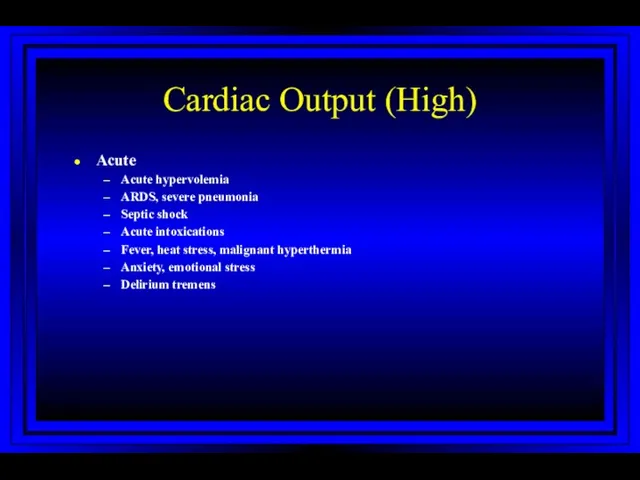
- 30. Cardiac Output (High) Acute Acute hypervolemia ARDS, severe pneumonia Septic shock Acute intoxications Fever, heat stress,
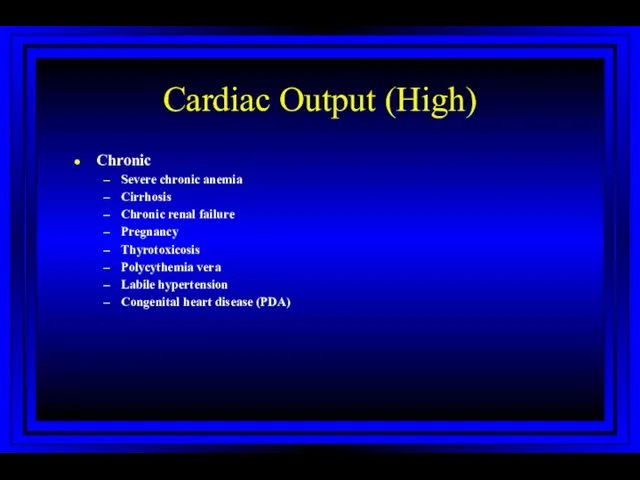
- 31. Cardiac Output (High) Chronic Severe chronic anemia Cirrhosis Chronic renal failure Pregnancy Thyrotoxicosis Polycythemia vera Labile
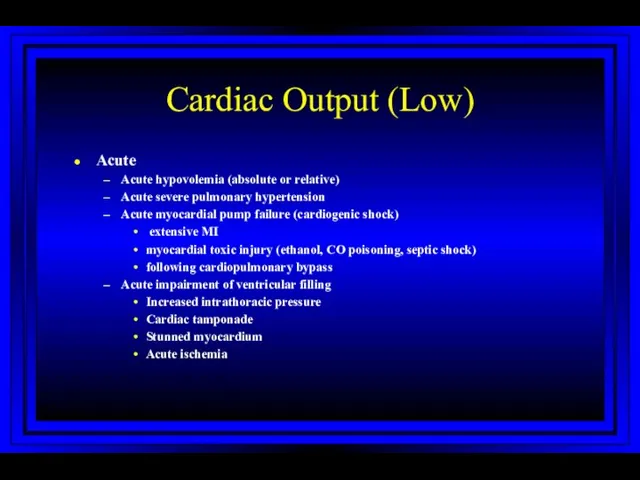
- 32. Cardiac Output (Low) Acute Acute hypovolemia (absolute or relative) Acute severe pulmonary hypertension Acute myocardial pump
- 33. Cardiac Output (Low) Acute Arrhythmias Sustained VT Extreme bradycardia Acute inotropic changes in a failing myocardium
- 34. Cardiac Output (Low) Chronic Chronic severe pulmonary hypertension Chronic myocardial pump failure Ischemia Hypertensive or dilated
- 35. Shunts Demonstrated by an absence of an expected pressure difference With a significant ASD the left
- 36. Shunts Evaluation of shunts requires: Detection Classification Localization Quantitation
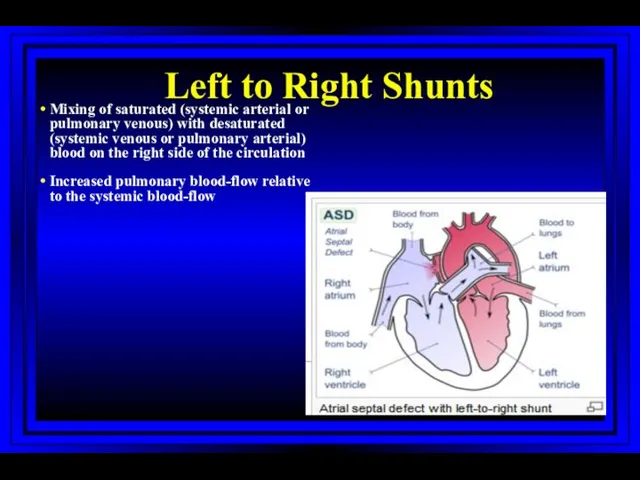
- 37. Left to Right Shunts Mixing of saturated (systemic arterial or pulmonary venous) with desaturated (systemic venous
- 38. Right to Left Shunts Mixing of desaturated (systemic venous or pulmonary arterial) with saturated (systemic arterial
- 39. Pulmonary Hypertension: Role of Right Heart Catheterization For diagnosis For evaluating acute vasodilator response For evaluating
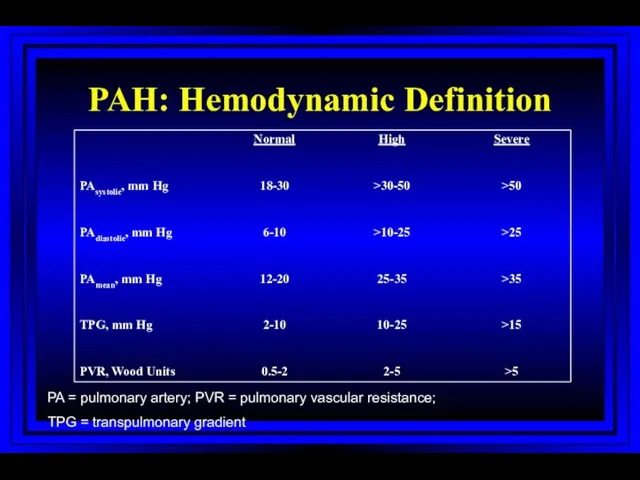
- 40. PAH: Hemodynamic Definition PA = pulmonary artery; PVR = pulmonary vascular resistance; TPG = transpulmonary gradient
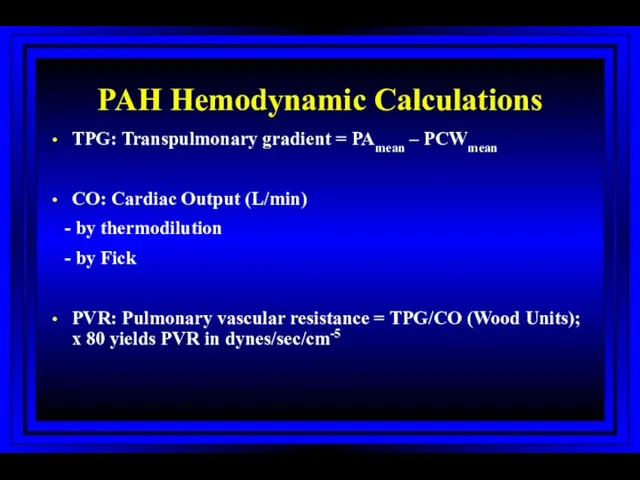
- 41. PAH Hemodynamic Calculations TPG: Transpulmonary gradient = PAmean – PCWmean CO: Cardiac Output (L/min) - by
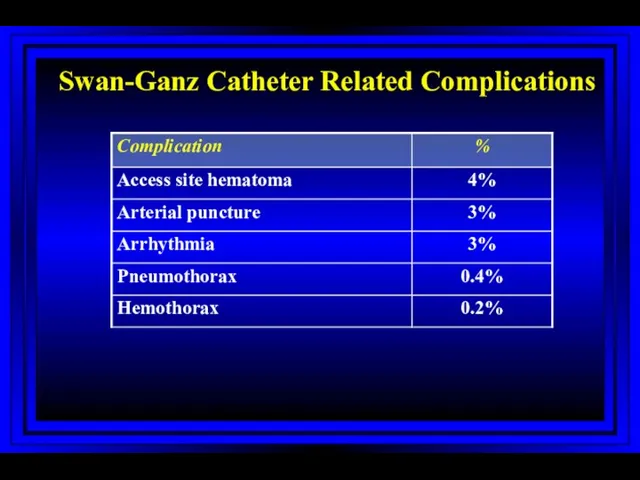
- 42. Swan-Ganz Catheter Related Complications Harvey S et al. The Lancet 2005; 366:472-477
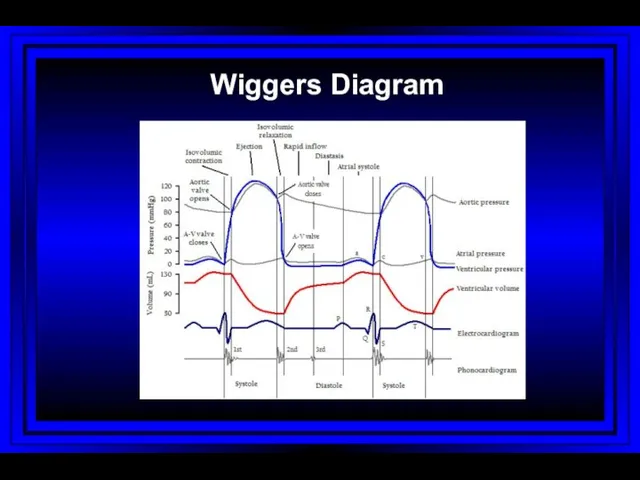
- 43. Wiggers Diagram
- 44. Left Heart Catheterization: History First human catheterization by Werner Forssmann: 1929 His work was not recognized
- 45. Vascular Access: Left Heart Cath Sones’ technique (brachial approach) Judkin’s technique (femoral approach) Radial approach
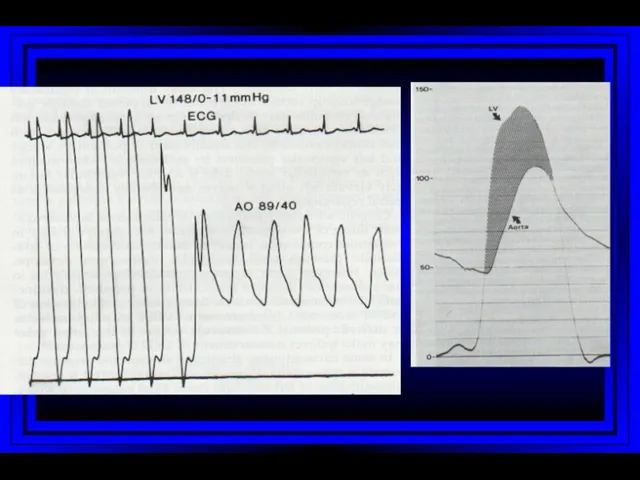
- 46. Left Heart Catheterization Coronary angiography Left ventriculogram Ascending aortogram Pressure measurements in LV/aorta
- 47. Cardiac Angiography: Ventriculography A contrast roadmap of the left ventricle allows for evaluation of: Ventricular chamber
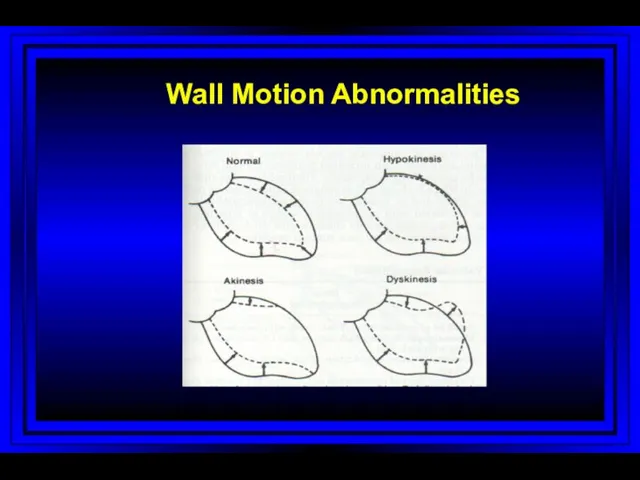
- 48. Wall Motion Abnormalities
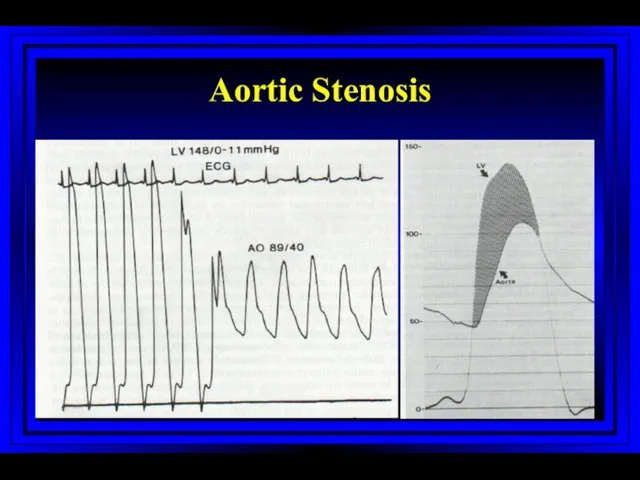
- 49. Aortic Stenosis
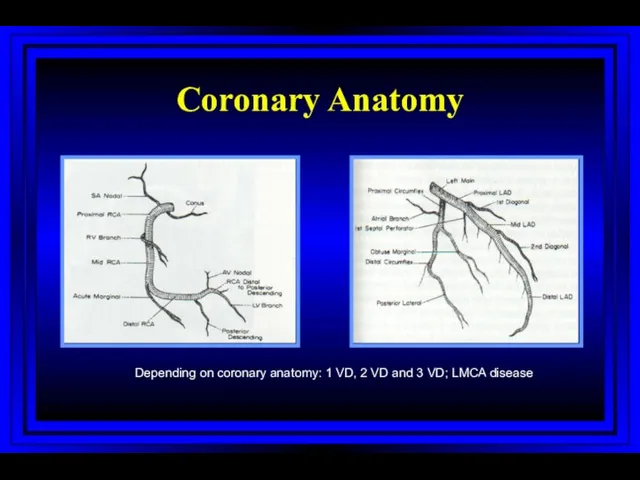
- 50. Coronary Anatomy Depending on coronary anatomy: 1 VD, 2 VD and 3 VD; LMCA disease mm
- 51. Treatment Strategies of CAD Medical treatment, PCI or CABG - for pts with distal CAD; risk
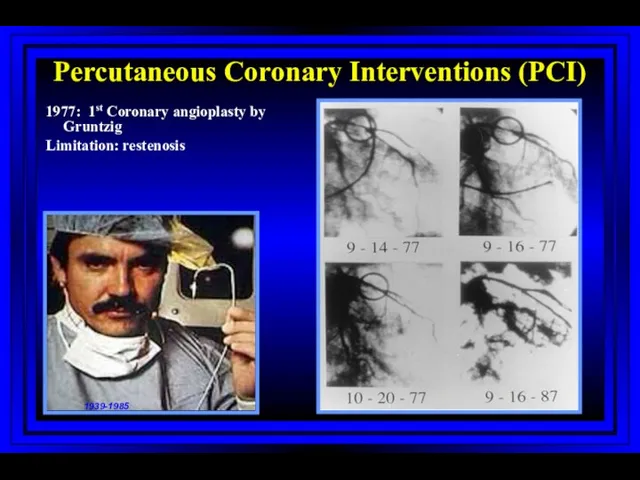
- 52. Percutaneous Coronary Interventions (PCI) 1977: 1st Coronary angioplasty by Gruntzig Limitation: restenosis 1939-1985
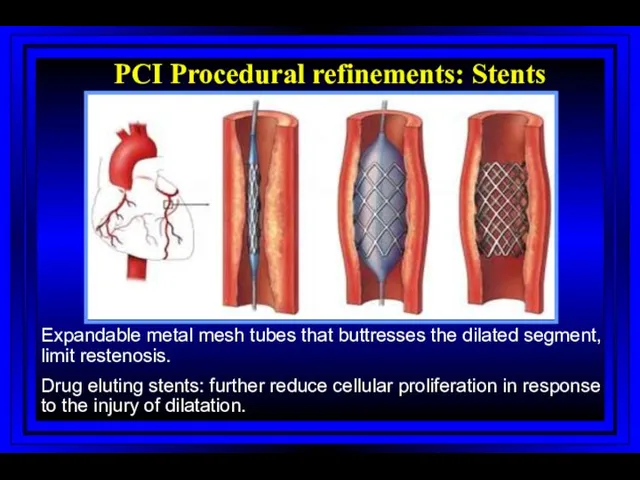
- 53. PCI Procedural refinements: Stents Expandable metal mesh tubes that buttresses the dilated segment, limit restenosis. Drug
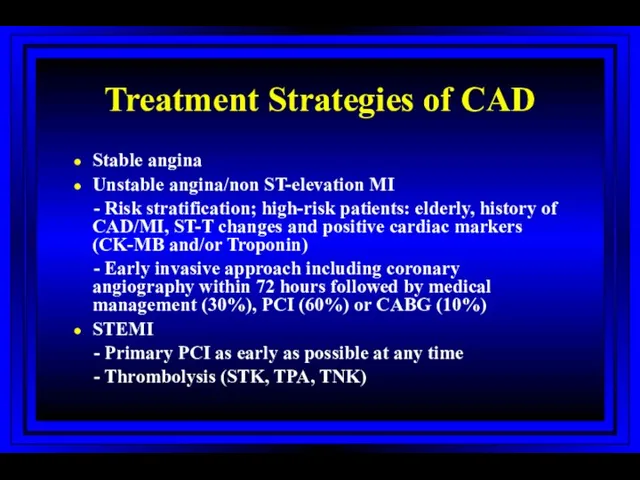
- 54. Treatment Strategies of CAD Stable angina Unstable angina/non ST-elevation MI - Risk stratification; high-risk patients: elderly,
- 55. Treatment Strategies of CAD Stable angina Unstable angina/non ST-elevation MI - Risk stratification; high-risk patients: elderly,
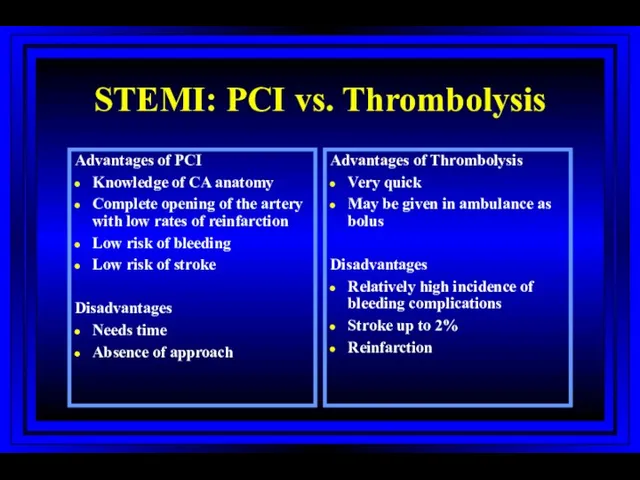
- 56. STEMI: PCI vs. Thrombolysis Advantages of PCI Knowledge of CA anatomy Complete opening of the artery
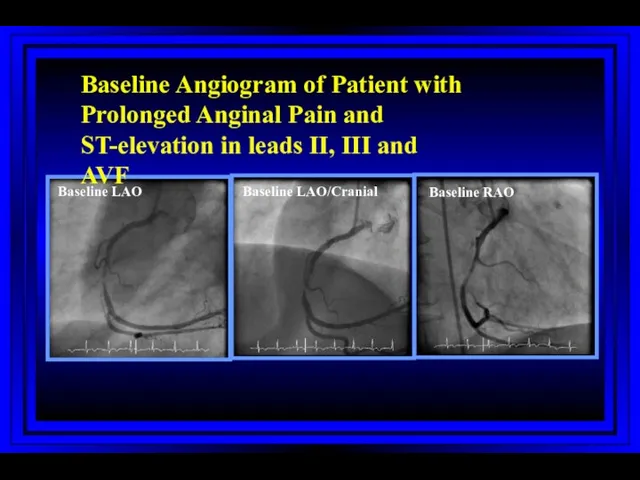
- 57. Baseline LAO Baseline LAO/Cranial Baseline RAO Baseline Angiogram of Patient with Prolonged Anginal Pain and ST-elevation
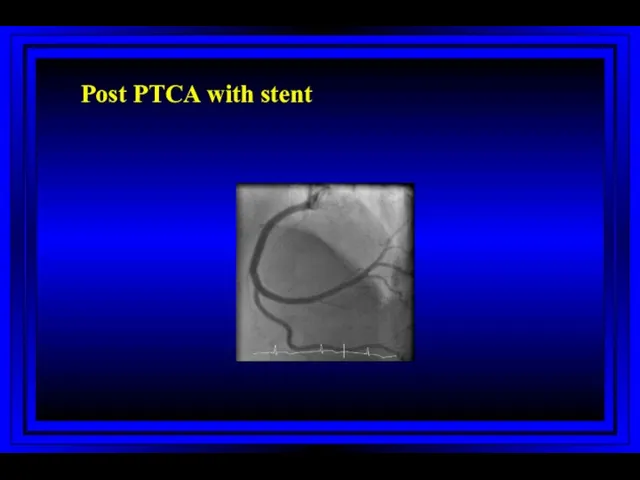
- 58. Post PTCA with stent
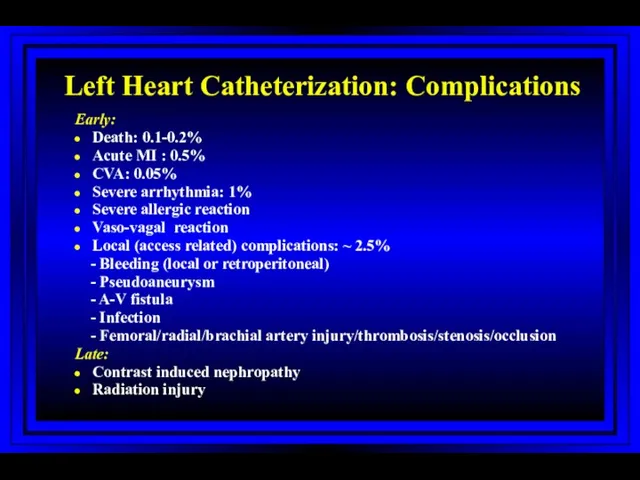
- 59. Left Heart Catheterization: Complications Early: Death: 0.1-0.2% Acute MI : 0.5% CVA: 0.05% Severe arrhythmia: 1%
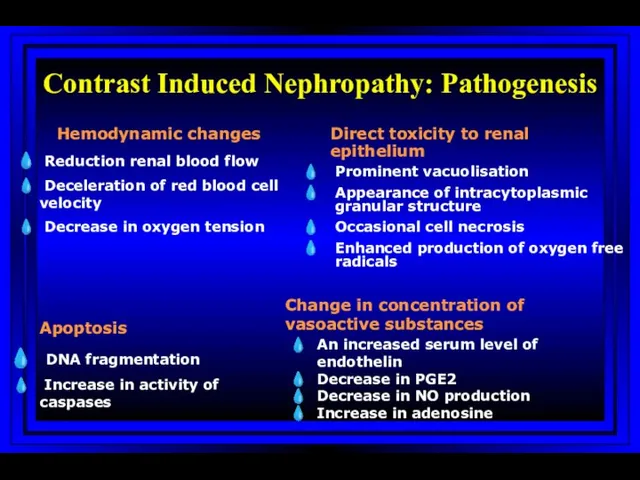
- 60. Contrast Induced Nephropathy: Pathogenesis Hemodynamic changes Reduction renal blood flow Deceleration of red blood cell velocity
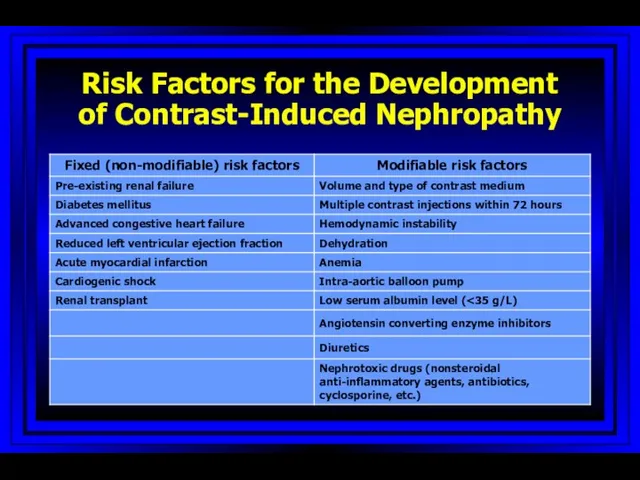
- 61. Risk Factors for the Development of Contrast-Induced Nephropathy
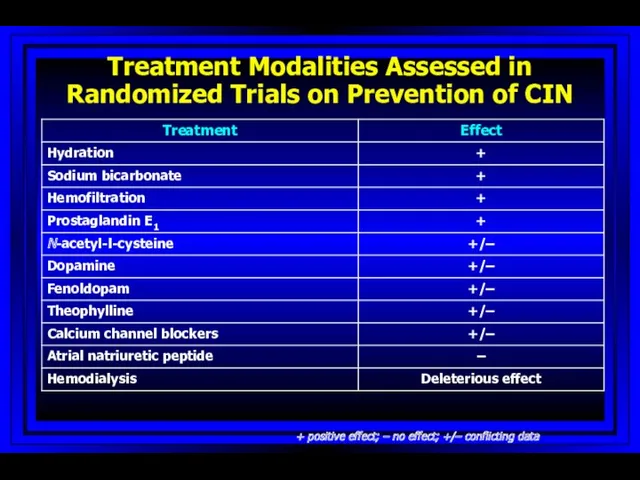
- 62. Treatment Modalities Assessed in Randomized Trials on Prevention of CIN + positive effect; – no effect;
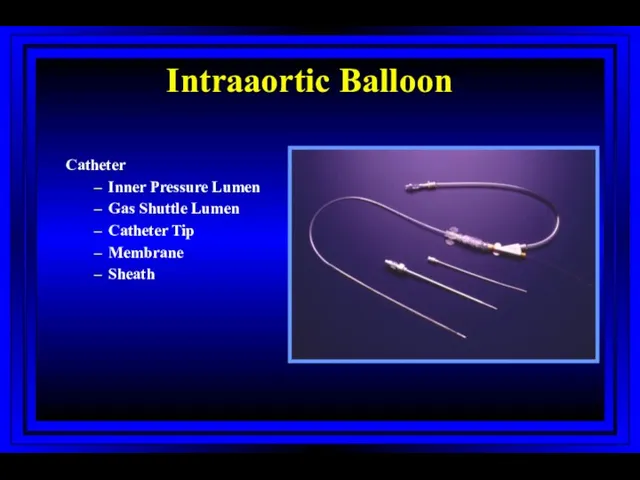
- 63. Intraaortic Balloon Catheter Inner Pressure Lumen Gas Shuttle Lumen Catheter Tip Membrane Sheath
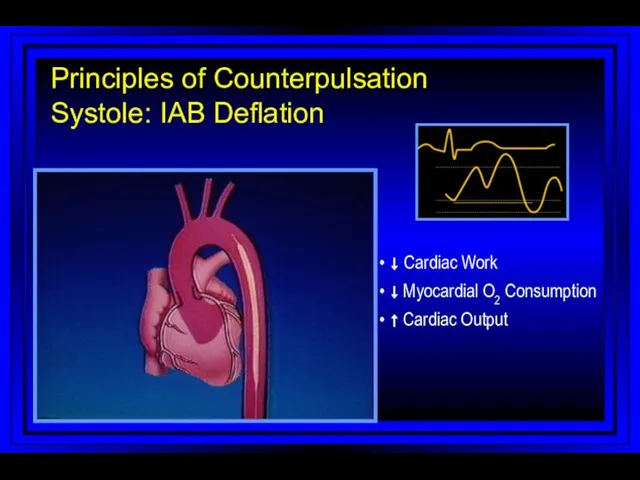
- 64. • ↓ Cardiac Work • ↓ Myocardial O2 Consumption • ↑ Cardiac Output Principles of Counterpulsation
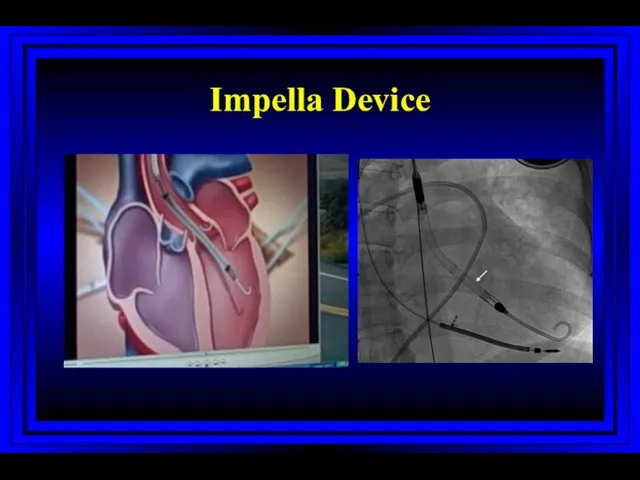
- 65. Impella Device
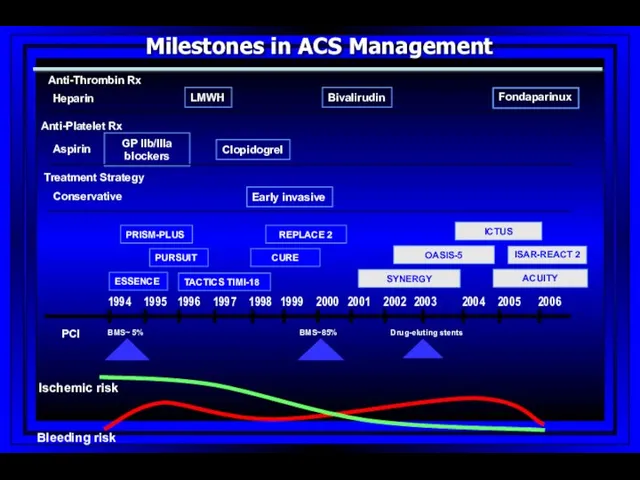
- 66. SYNERGY 1994 1995 1996 1997 1998 1999 2000 2002 2003 2004 2005 2006 2001 Bleeding risk
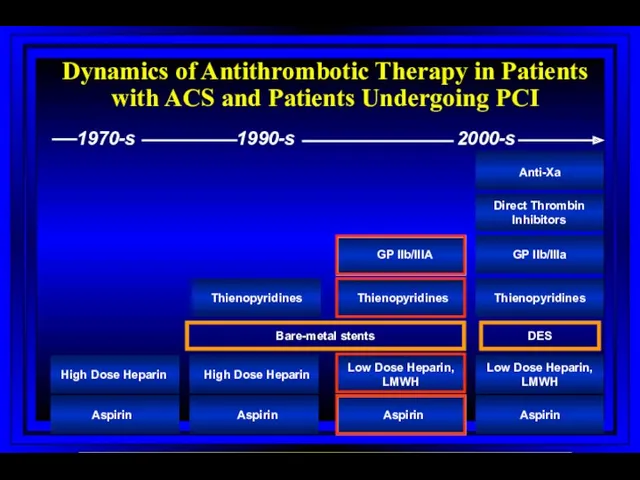
- 67. Dynamics of Antithrombotic Therapy in Patients with ACS and Patients Undergoing PCI Aspirin Aspirin Aspirin Aspirin
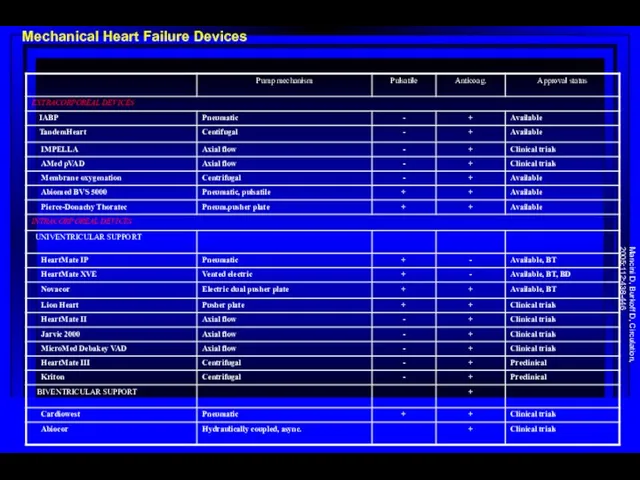
- 68. Mechanical Heart Failure Devices Mancini D, Burkoff D, Circulation, 2005;112:438-446
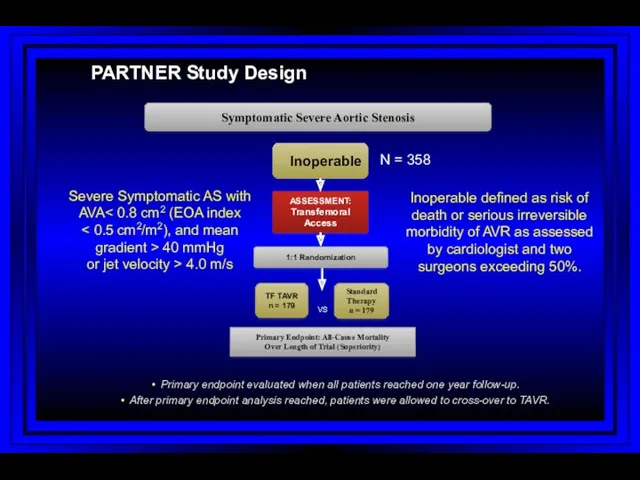
- 69. PARTNER Study Design N = 358 Inoperable Standard Therapy n = 179 ASSESSMENT: Transfemoral Access TF
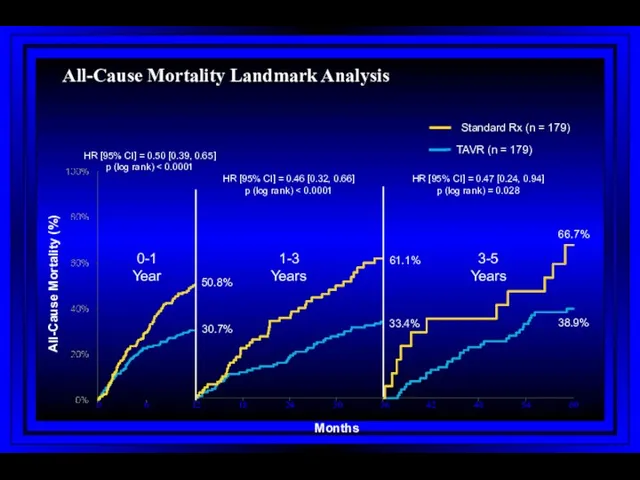
- 70. All-Cause Mortality Landmark Analysis
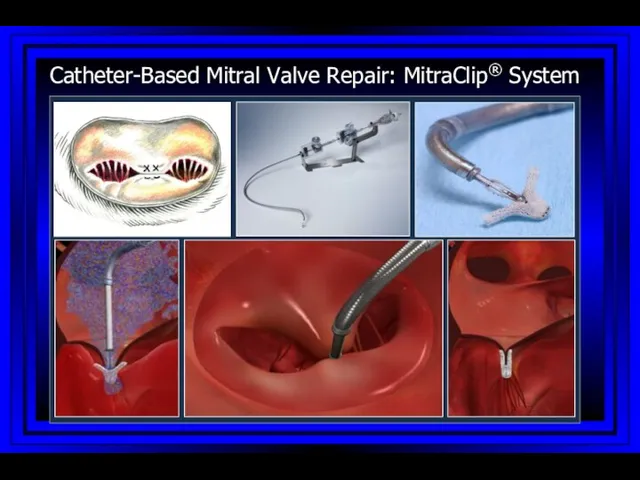
- 71. Catheter-Based Mitral Valve Repair: MitraClip® System
- 72. Investigational Device only in the US; Not available for sale in the US EVEREST II Randomized
- 74. Скачать презентацию














 Ортодонтическое лечение. Пациент
Ортодонтическое лечение. Пациент Клапанная болезнь сердца и беременность
Клапанная болезнь сердца и беременность Логопедическая работа
Логопедическая работа Острые отравления этанолом
Острые отравления этанолом Сосудистые анастомозы РУС
Сосудистые анастомозы РУС Выделительная система человека
Выделительная система человека Организация деятельности аптечных организаций
Организация деятельности аптечных организаций Дәрілік өсімдіктер
Дәрілік өсімдіктер Зрительные функции и методы их исследования
Зрительные функции и методы их исследования Сахарный диабет I типа
Сахарный диабет I типа Рак слизистой оболочки полости рта
Рак слизистой оболочки полости рта О передаче медицинскими организациями электронных медицинских документов в РЭМД ЕГИСЗ
О передаче медицинскими организациями электронных медицинских документов в РЭМД ЕГИСЗ Мысқыл (паротит)
Мысқыл (паротит) Сосудистый шов
Сосудистый шов Ургентная помощь при острой сердечной и острой сосудистой недостаточности. Обморок, коллапс, шок
Ургентная помощь при острой сердечной и острой сосудистой недостаточности. Обморок, коллапс, шок Нарушения кислотно-основного состояния
Нарушения кислотно-основного состояния Детский хоспис Дом с маяком
Детский хоспис Дом с маяком Гомеопатия
Гомеопатия Методы диагностики гипертрофии миокарда
Методы диагностики гипертрофии миокарда Жүктіліктің УД зерттеу әдісі
Жүктіліктің УД зерттеу әдісі Система непрерывного профессионального развития среднего медицинского персонала
Система непрерывного профессионального развития среднего медицинского персонала Частная микробиология
Частная микробиология Общие принципы микробиологической диагностики кишечной группы бактерии. Постановка этиологического диагноза
Общие принципы микробиологической диагностики кишечной группы бактерии. Постановка этиологического диагноза Витаминные добавки в парфюмерно-косметической промышленности
Витаминные добавки в парфюмерно-косметической промышленности Балалардың жақ-бет аймағындағы деформациялар мен ақауларды емдеудің негізгі әдістері
Балалардың жақ-бет аймағындағы деформациялар мен ақауларды емдеудің негізгі әдістері Бүйрек, несепағар жарақаты
Бүйрек, несепағар жарақаты Клинико-фармакологическая характеристика лекарств, влияющих на моторно-секреторную функцию желудочно-кишечного тракта
Клинико-фармакологическая характеристика лекарств, влияющих на моторно-секреторную функцию желудочно-кишечного тракта Всесвітній день боротьби зі СНІДом
Всесвітній день боротьби зі СНІДом