Содержание
- 2. Plan Authorship, confidentiality, etc. Citation Etiquette Misappropriation of Ideas Citing The Source of an Idea Responsibilities
- 3. Ethics Ethics – the discipline concerned with what is morally good and bad, right and wrong
- 4. Definition of Scientific Misconduct Scientific misconduct is fabrication, falsification, or plagiarism in proposing, performing, or reviewing
- 5. Codes and guidelines evolved because of human subjects’ rights abuses Nazi experiments using war chemicals, environmental
- 6. GENERAL BASIC PRINCIPALS OF ETHICS: 1. Honesty : Honestly report data ,results ,methods and procedures and
- 7. 1. Why is ethical problems important? Ethical discussions usually remain detached or marginalized from discussions of
- 8. What responsibility do you have toward your research subjects? The term ethics derives from the Greek
- 9. What responsibility do you have toward your research subjects? What responsibility do you have toward your
- 10. A consideration of ethics needs to be a critical part of the substructure of the research
- 11. Codes and Guidelines 1974 – US Congress formed the National Commission for the Protection of Human
- 12. Further Developments in the History of Research Ethics Formal consideration of the rights of research subjects
- 13. the Declaration of Helsinki (1964), Other codes of ethics soon followed, including the Declaration of Helsinki
- 14. Throughout the history of scientific research, ethical issues have captured the attention of scientists and the
- 16. Plagiarism Plagiarism—using the ideas, writings, and drawings of others as your own
- 17. Fabrication and Falsification Fabrication and falsification—making up or altering data
- 18. Researcher Faces Prison for Fraud in NIH Grant Applications and Papers Science 25 March 2005: Vol.
- 19. Nonpublication of Data Sometimes called “cooking data” Data not included in results because they don’t support
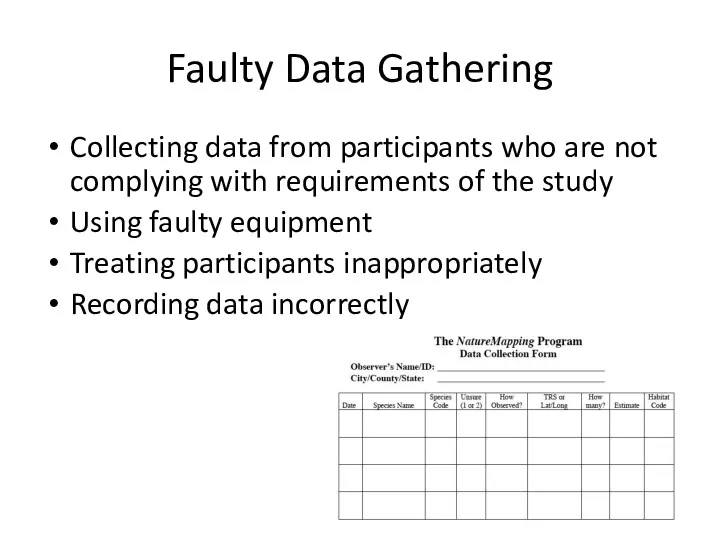
- 20. Faulty Data Gathering Collecting data from participants who are not complying with requirements of the study
- 21. Data Gathering Most important and most aggravating. Always drop non-compliers. Fix broken equipment. Treat subjects with
- 22. Poor Data Storage and Retention Data should be stored in its original collected form for at
- 23. Misleading Authorship Misleading authorship—who should be an author? Technicians do not necessarily become joint authors. Authorship
- 24. MSSE Information for Authors Medicine & Science in Sports & Exercise® Authorship Requirements To be an
- 25. Sneaky Publication Practices Publication of the thesis or dissertation Should be regarded as the student’s work
- 26. Sanctions Freeze your job. Reduce your job. Lose your job. Loss of institution money and privileges.
- 27. The Common Rule mandated, among other things, that any institution receiving federal funds for research must
- 28. They must determine whether the benefits of a study outweigh its risks, whether consent procedures have
- 29. Academic Etiquette For some reason, academics are not particularly famous for having well-developed social skills, although
- 30. For reviewers: When writing a review, even if you think the authors are wrong or have
- 31. 6. For professors: If you don't like another professor, don't take your dislike out on their
- 32. The awkwardness and occasional hostility that may arise among scholars in competitive fields gets even more
- 33. Don't make faculty meetings last longer than necessary unless you have something really important to say.
- 35. Скачать презентацию































 Элективные курсы по физической культуре и спорту
Элективные курсы по физической культуре и спорту ФГОС второго поколения: состав и содержание основных документов стандарта
ФГОС второго поколения: состав и содержание основных документов стандарта Обучающиеся с ограниченными возможностями здоровья и дети-инвалиды: различие понятий
Обучающиеся с ограниченными возможностями здоровья и дети-инвалиды: различие понятий Домашнее задание как средство формирования прочных учебных компетентностей
Домашнее задание как средство формирования прочных учебных компетентностей Методология подготовки и написания диссертации
Методология подготовки и написания диссертации Создание клуба интеллектуальных игр
Создание клуба интеллектуальных игр 320 орындық жаңа үлгідегі мектеп
320 орындық жаңа үлгідегі мектеп Конкурс Лучший класс. Программа Мотив
Конкурс Лучший класс. Программа Мотив Advanced Research Projects. Winter Semester 2015 / 2016
Advanced Research Projects. Winter Semester 2015 / 2016 Компетенции местного самоуправления в стратегическом управлении развития
Компетенции местного самоуправления в стратегическом управлении развития Новый УМК по русскому языку для 5-9 классов основной общеобразовательной школы
Новый УМК по русскому языку для 5-9 классов основной общеобразовательной школы Формирование познавательных УУД на уроках математики
Формирование познавательных УУД на уроках математики презентация Родословная моей семьи
презентация Родословная моей семьи Современные воспитательные технологии.
Современные воспитательные технологии. Процес вступу до американських коледжів та університетів та фінансова допомога. Мережа консультативних центрів EducationUSA
Процес вступу до американських коледжів та університетів та фінансова допомога. Мережа консультативних центрів EducationUSA Пути повышения качества естественно-научного образования
Пути повышения качества естественно-научного образования Как написать исследовательскую работу
Как написать исследовательскую работу проектирование рабочей программы по русскому языку при переходе на ФГОС
проектирование рабочей программы по русскому языку при переходе на ФГОС Как создать и оформить проект
Как создать и оформить проект Учебная программа по предмету Русский язык и литература
Учебная программа по предмету Русский язык и литература Сибирский федеральный университет. Специальности, направления и профили. Поступление
Сибирский федеральный университет. Специальности, направления и профили. Поступление Объединённый совет обучающихся (ОСО)
Объединённый совет обучающихся (ОСО) Интерактивнообразовательный КВИЗ
Интерактивнообразовательный КВИЗ Работа с одаренными детьми в условиях общеобразовательной школы
Работа с одаренными детьми в условиях общеобразовательной школы Антитеррористическая защищенность образовательных организаций
Антитеррористическая защищенность образовательных организаций Презентация Обереги - берегини.
Презентация Обереги - берегини. Абай Кунанбаев
Абай Кунанбаев План работы Районного Методического Объединения учителей истории Диск Диск
План работы Районного Методического Объединения учителей истории Диск Диск