Содержание
- 2. Overview: The Energy of Life The living cell is a miniature chemical factory where thousands of
- 3. Fig. 8-1
- 4. Concept 8.1: An organism’s metabolism transforms matter and energy, subject to the laws of thermodynamics Metabolism
- 5. Organization of the Chemistry of Life into Metabolic Pathways A metabolic pathway begins with a specific
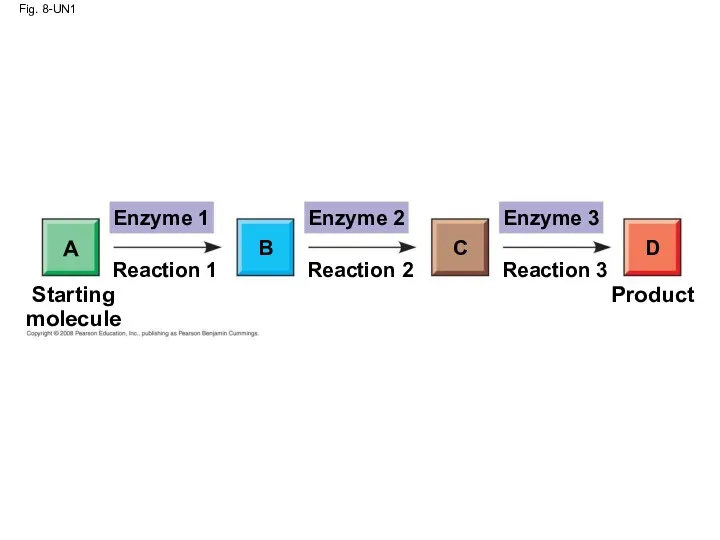
- 6. Fig. 8-UN1 Enzyme 1 Enzyme 2 Enzyme 3 D C B A Reaction 1 Reaction 3
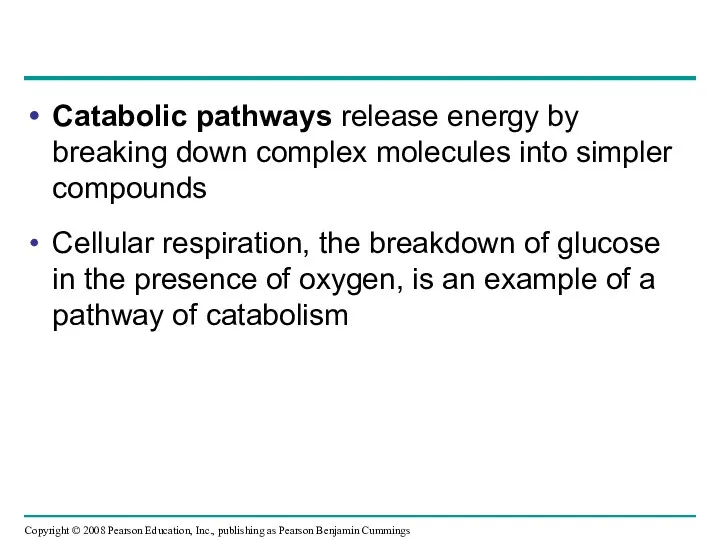
- 7. Catabolic pathways release energy by breaking down complex molecules into simpler compounds Cellular respiration, the breakdown
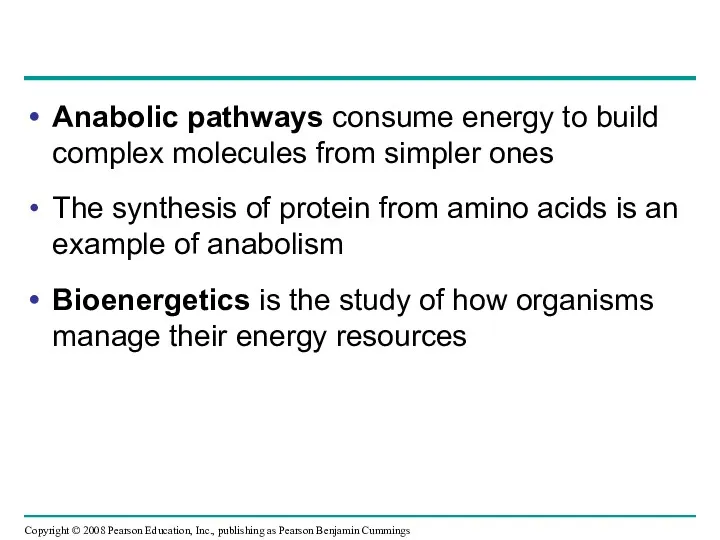
- 8. Anabolic pathways consume energy to build complex molecules from simpler ones The synthesis of protein from
- 9. Forms of Energy Energy is the capacity to cause change Energy exists in various forms, some
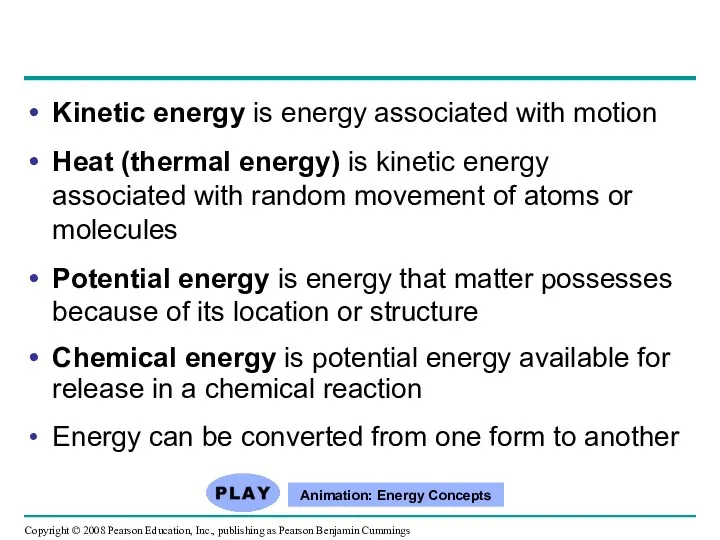
- 10. Kinetic energy is energy associated with motion Heat (thermal energy) is kinetic energy associated with random
- 11. Fig. 8-2 Climbing up converts the kinetic energy of muscle movement to potential energy. A diver
- 12. The Laws of Energy Transformation Thermodynamics is the study of energy transformations A closed system, such
- 13. The First Law of Thermodynamics According to the first law of thermodynamics, the energy of the
- 14. The Second Law of Thermodynamics During every energy transfer or transformation, some energy is unusable, and
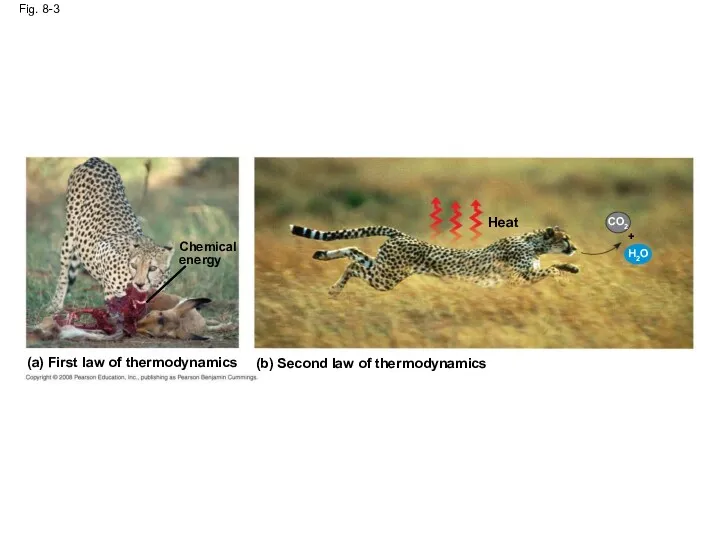
- 15. Fig. 8-3 (a) First law of thermodynamics (b) Second law of thermodynamics Chemical energy Heat CO2
- 16. Living cells unavoidably convert organized forms of energy to heat Spontaneous processes occur without energy input;
- 17. Biological Order and Disorder Cells create ordered structures from less ordered materials Organisms also replace ordered
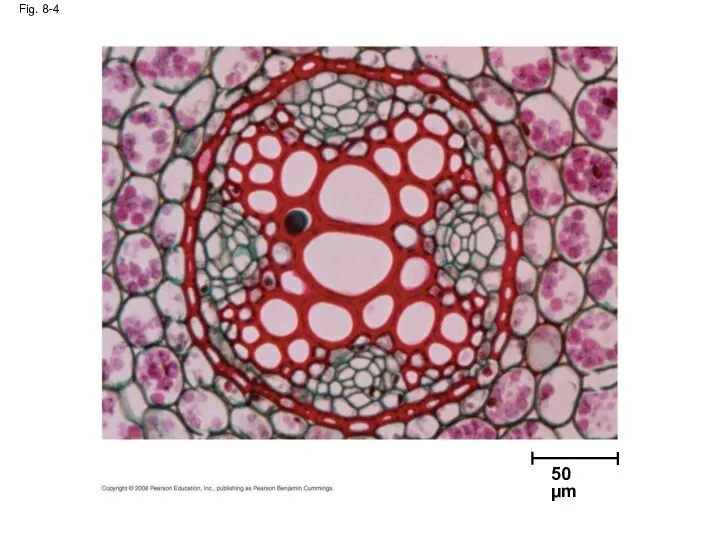
- 18. Fig. 8-4 50 µm
- 19. The evolution of more complex organisms does not violate the second law of thermodynamics Entropy (disorder)
- 20. Concept 8.2: The free-energy change of a reaction tells us whether or not the reaction occurs
- 21. Free-Energy Change, ΔG A living system’s free energy is energy that can do work when temperature
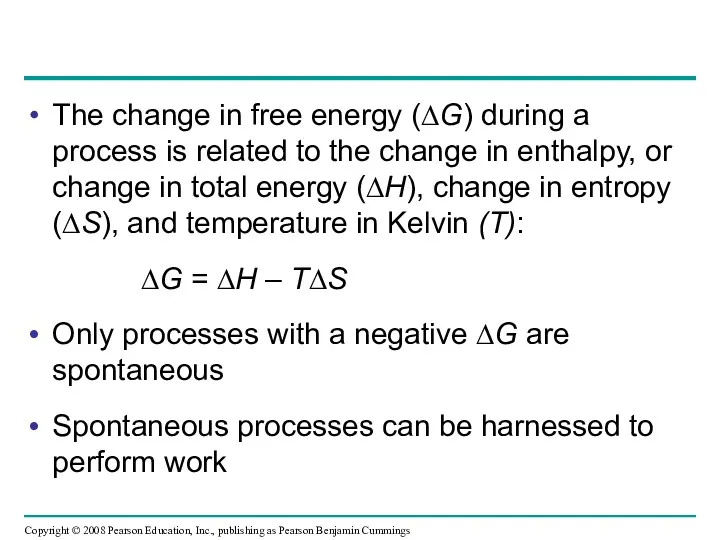
- 22. The change in free energy (∆G) during a process is related to the change in enthalpy,
- 23. Free Energy, Stability, and Equilibrium Free energy is a measure of a system’s instability, its tendency
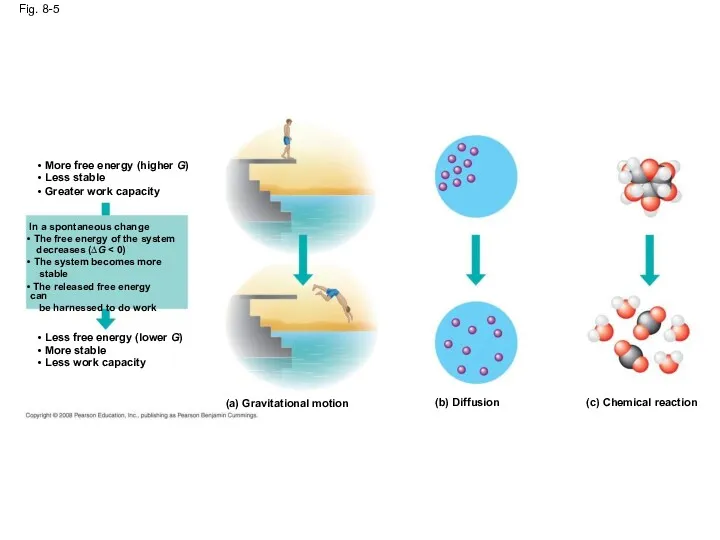
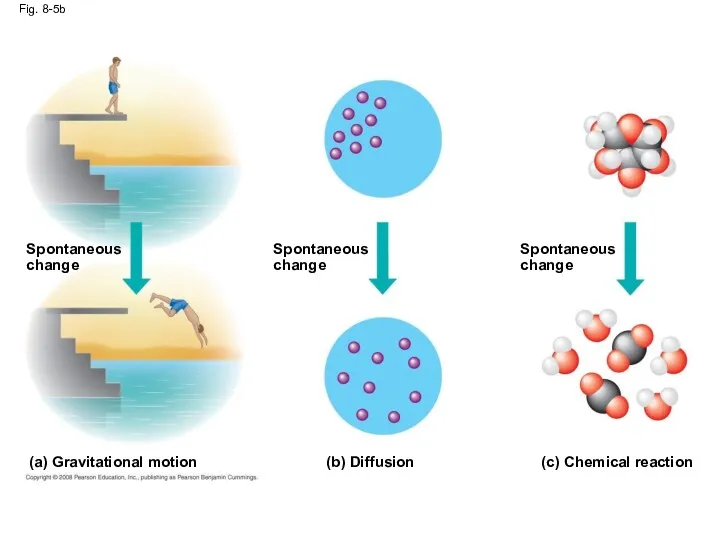
- 24. Fig. 8-5 (a) Gravitational motion (b) Diffusion (c) Chemical reaction More free energy (higher G) Less
- 25. Fig. 8-5a Less free energy (lower G) More stable Less work capacity More free energy (higher
- 26. Fig. 8-5b Spontaneous change Spontaneous change Spontaneous change (b) Diffusion (c) Chemical reaction (a) Gravitational motion
- 27. Free Energy and Metabolism The concept of free energy can be applied to the chemistry of
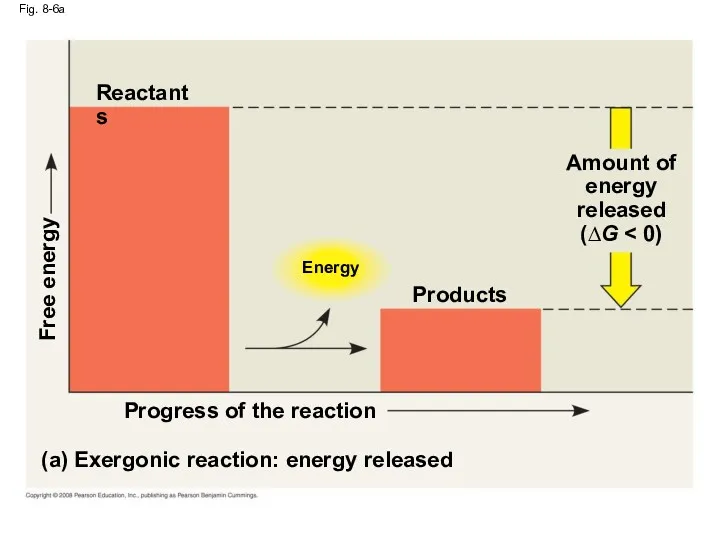
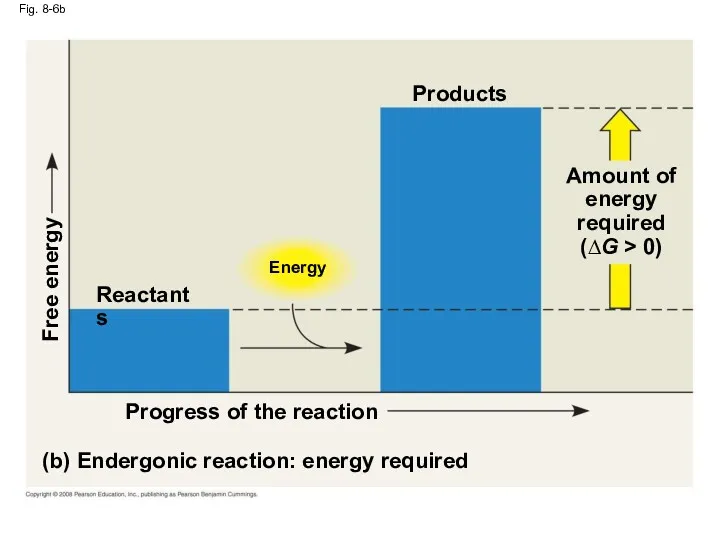
- 28. Exergonic and Endergonic Reactions in Metabolism An exergonic reaction proceeds with a net release of free
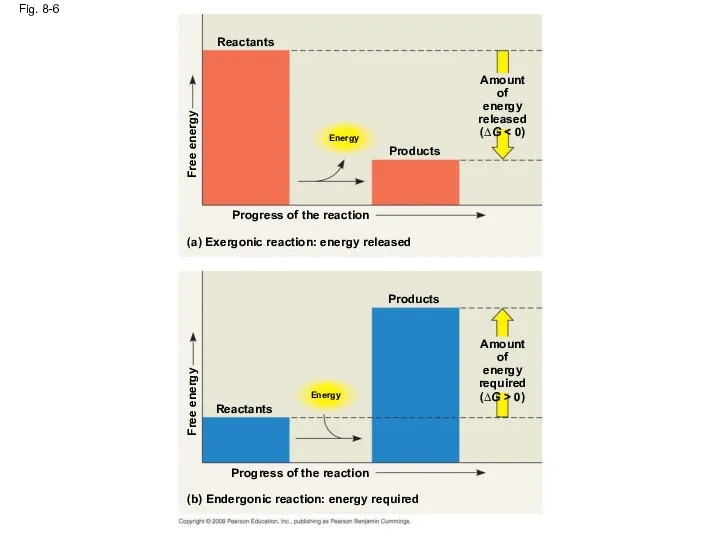
- 29. Fig. 8-6 Reactants Energy Free energy Products Amount of energy released (∆G Progress of the reaction
- 30. Fig. 8-6a Energy (a) Exergonic reaction: energy released Progress of the reaction Free energy Products Amount
- 31. Fig. 8-6b Energy (b) Endergonic reaction: energy required Progress of the reaction Free energy Products Amount
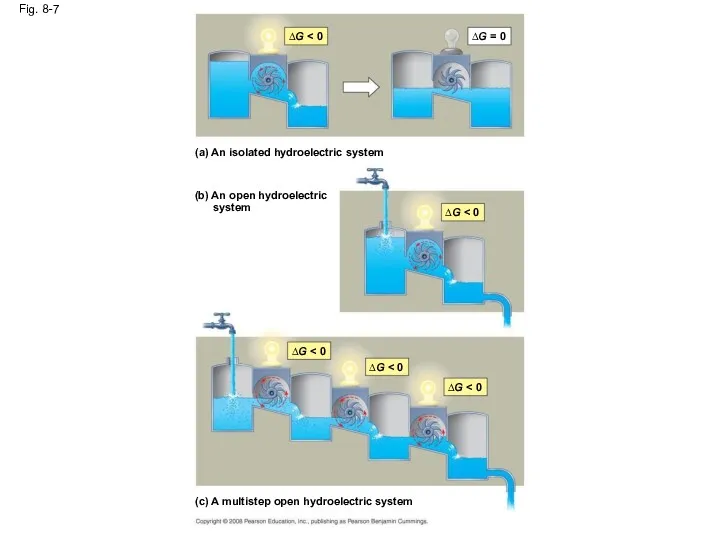
- 32. Equilibrium and Metabolism Reactions in a closed system eventually reach equilibrium and then do no work
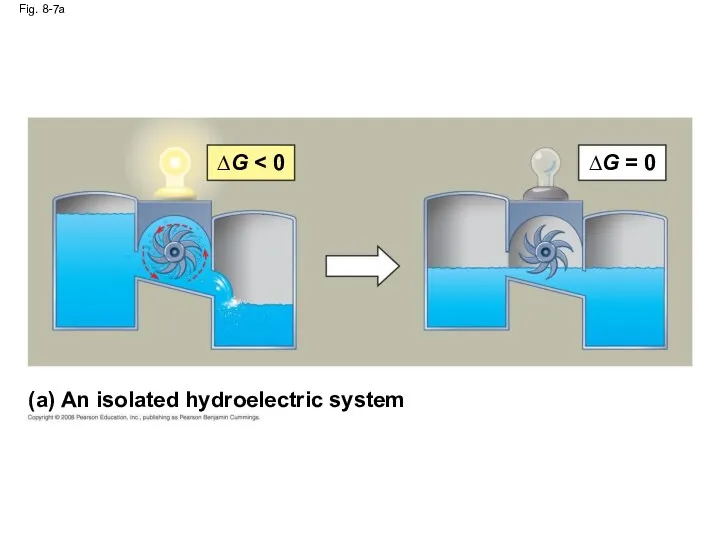
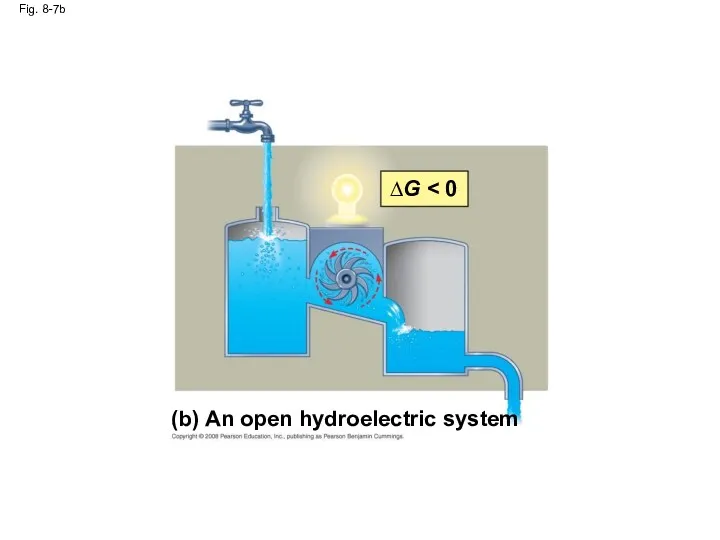
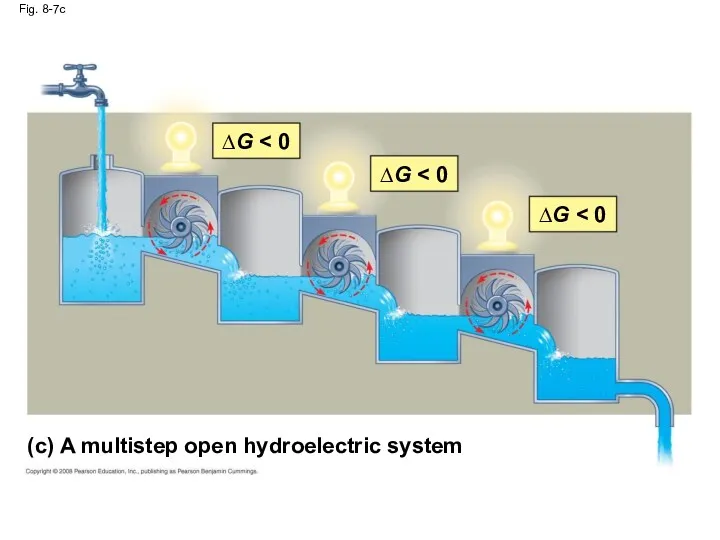
- 33. Fig. 8-7 (a) An isolated hydroelectric system ∆G ∆G = 0 (b) An open hydroelectric system
- 34. Fig. 8-7a (a) An isolated hydroelectric system ∆G ∆G = 0
- 35. Fig. 8-7b (b) An open hydroelectric system ∆G
- 36. Fig. 8-7c (c) A multistep open hydroelectric system ∆G ∆G ∆G
- 37. Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does
- 38. The Structure and Hydrolysis of ATP ATP (adenosine triphosphate) is the cell’s energy shuttle ATP is
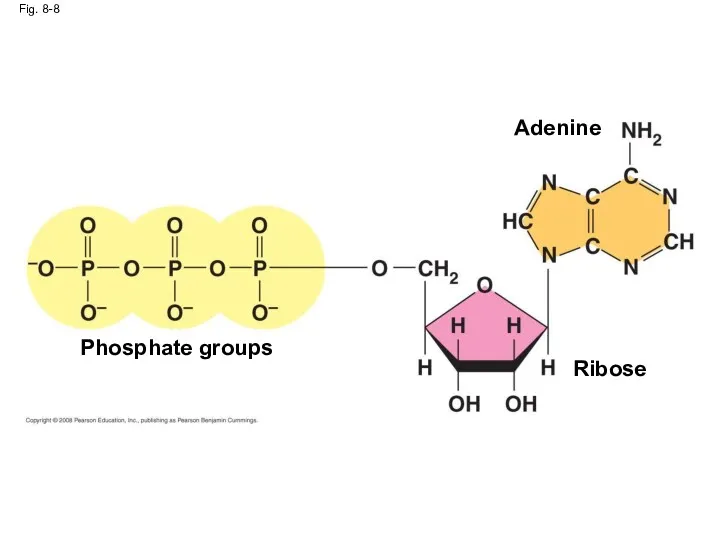
- 39. Fig. 8-8 Phosphate groups Ribose Adenine
- 40. The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis Energy is
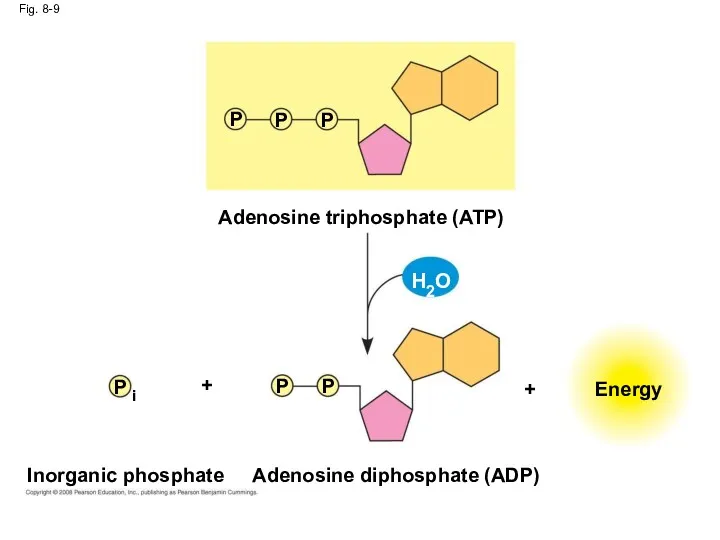
- 41. Fig. 8-9 Inorganic phosphate Energy Adenosine triphosphate (ATP) Adenosine diphosphate (ADP) P P P P P
- 42. How ATP Performs Work The three types of cellular work (mechanical, transport, and chemical) are powered
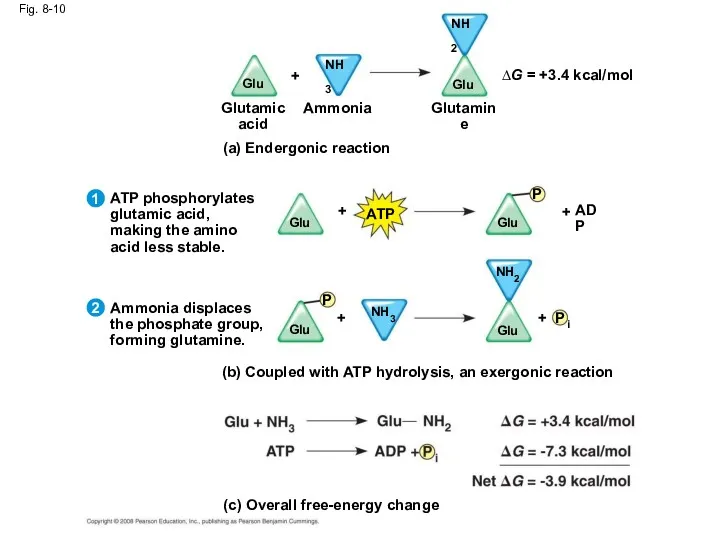
- 43. Fig. 8-10 (b) Coupled with ATP hydrolysis, an exergonic reaction Ammonia displaces the phosphate group, forming
- 44. ATP drives endergonic reactions by phosphorylation, transferring a phosphate group to some other molecule, such as
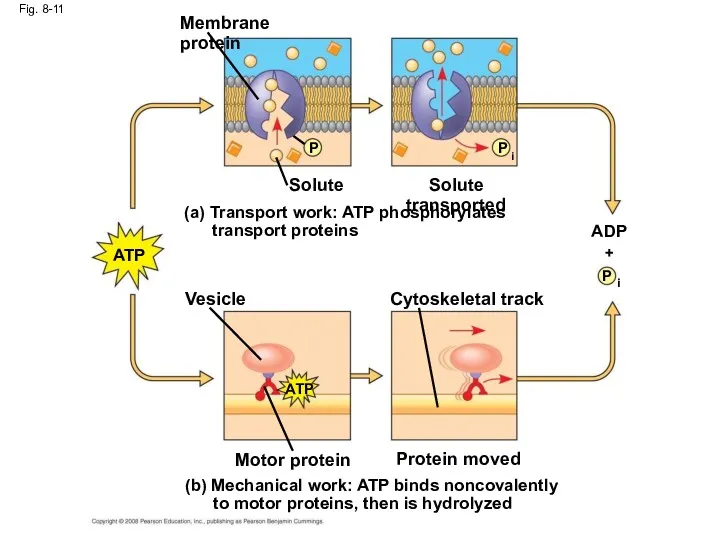
- 45. Fig. 8-11 (b) Mechanical work: ATP binds noncovalently to motor proteins, then is hydrolyzed Membrane protein
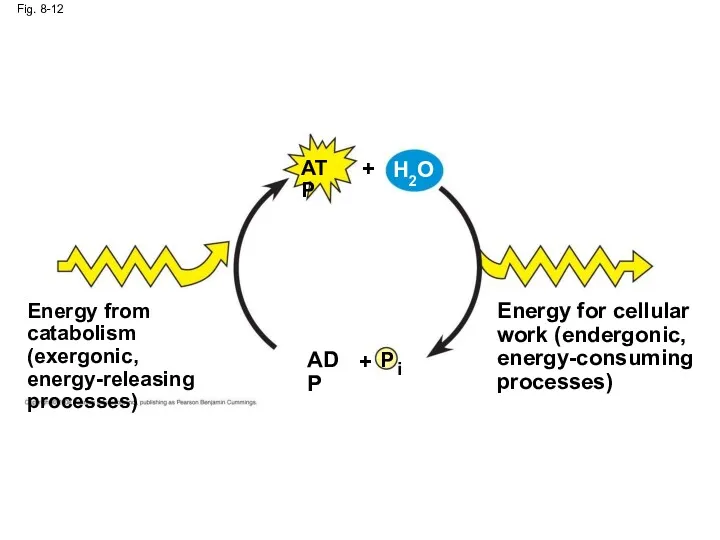
- 46. The Regeneration of ATP ATP is a renewable resource that is regenerated by addition of a
- 47. Fig. 8-12 P i ADP + Energy from catabolism (exergonic, energy-releasing processes) Energy for cellular work
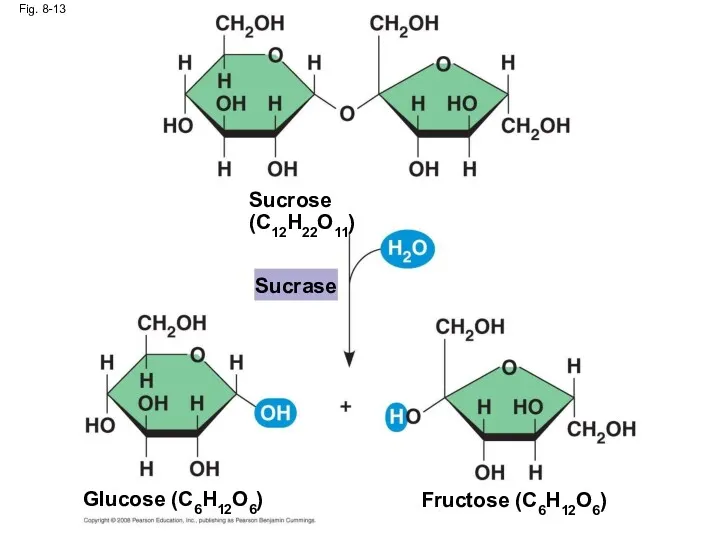
- 48. Concept 8.4: Enzymes speed up metabolic reactions by lowering energy barriers A catalyst is a chemical
- 49. Fig. 8-13 Sucrose (C12H22O11) Glucose (C6H12O6) Fructose (C6H12O6) Sucrase
- 50. The Activation Energy Barrier Every chemical reaction between molecules involves bond breaking and bond forming The
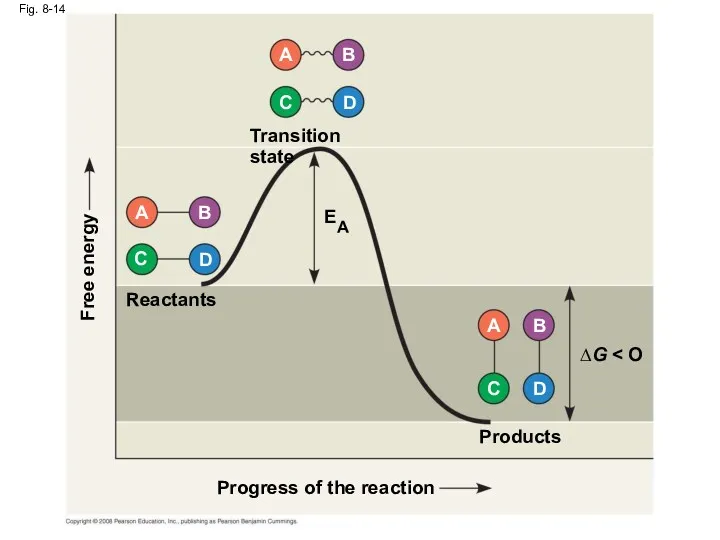
- 51. Fig. 8-14 Progress of the reaction Products Reactants ∆G Transition state Free energy EA D C
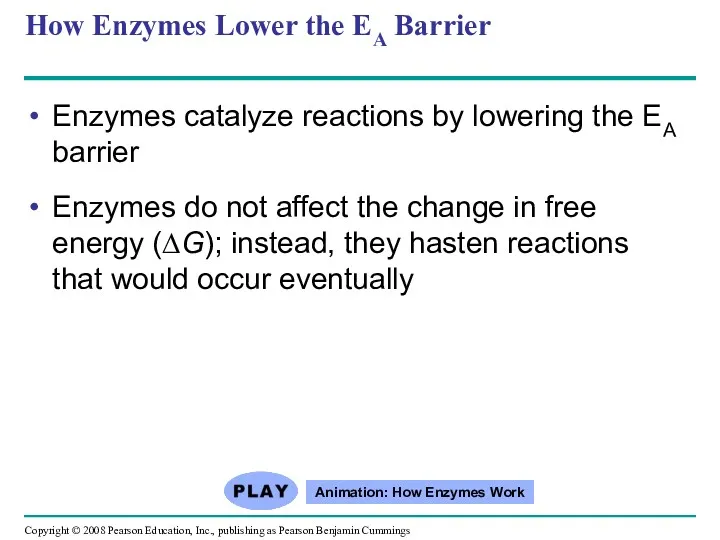
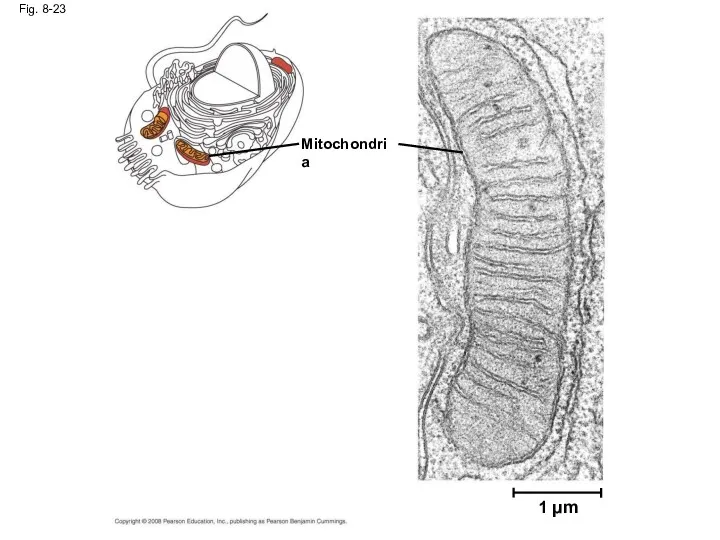
- 52. How Enzymes Lower the EA Barrier Enzymes catalyze reactions by lowering the EA barrier Enzymes do
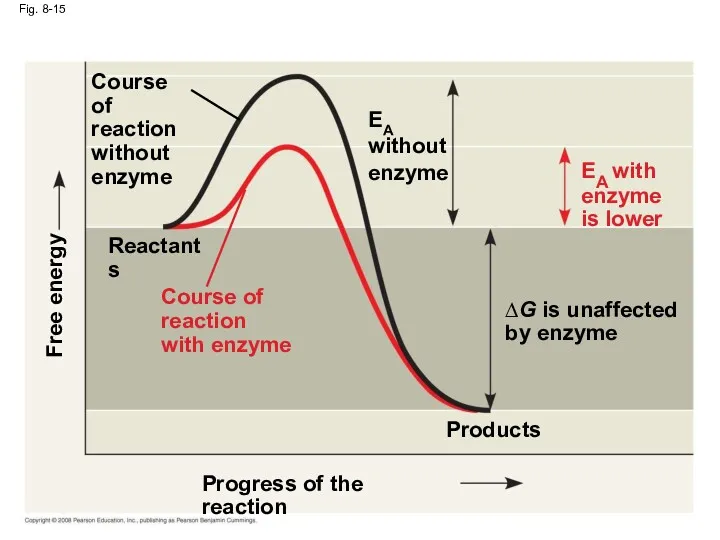
- 53. Fig. 8-15 Progress of the reaction Products Reactants ∆G is unaffected by enzyme Course of reaction
- 54. Substrate Specificity of Enzymes The reactant that an enzyme acts on is called the enzyme’s substrate
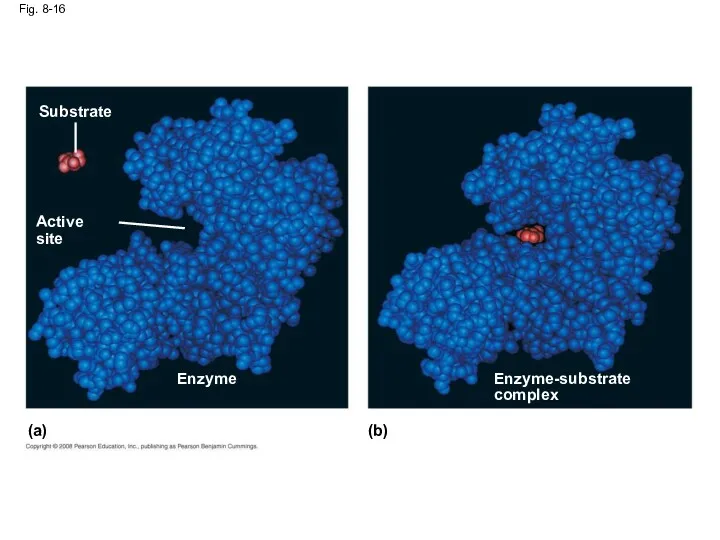
- 55. Fig. 8-16 Substrate Active site Enzyme Enzyme-substrate complex (b) (a)
- 56. Catalysis in the Enzyme’s Active Site In an enzymatic reaction, the substrate binds to the active
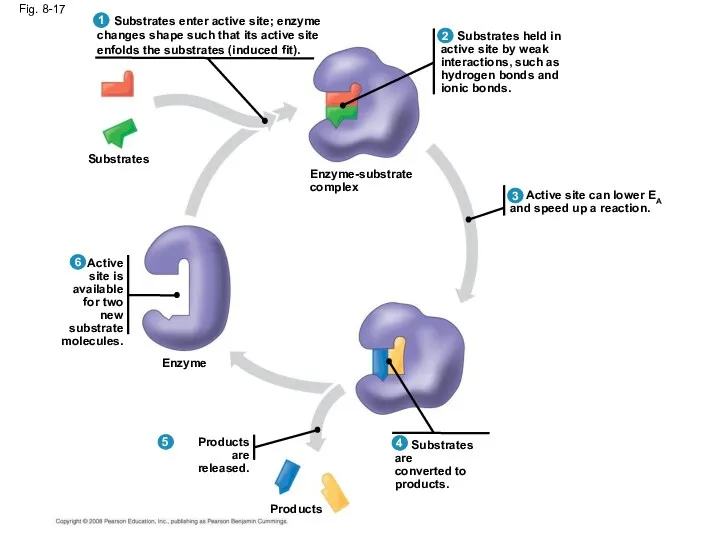
- 57. Fig. 8-17 Substrates Enzyme Products are released. Products Substrates are converted to products. Active site can
- 58. Effects of Local Conditions on Enzyme Activity An enzyme’s activity can be affected by General environmental
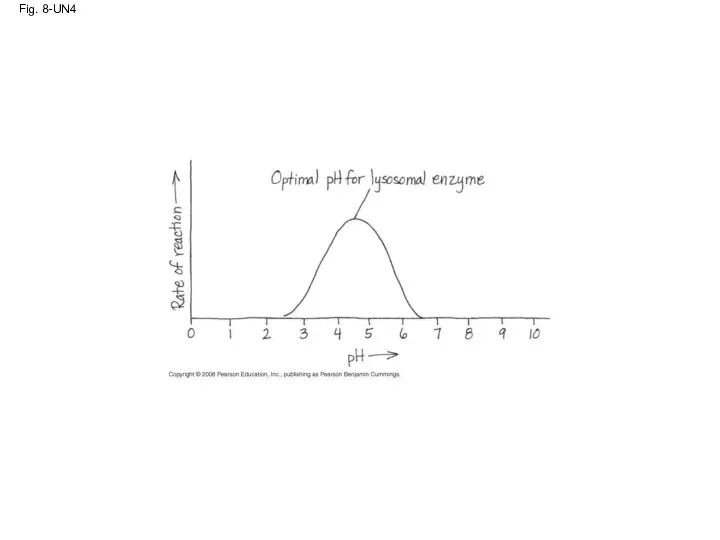
- 59. Effects of Temperature and pH Each enzyme has an optimal temperature in which it can function
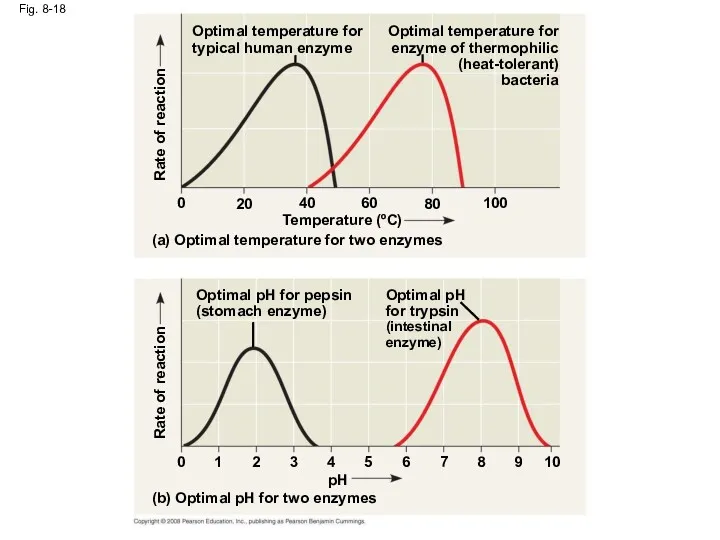
- 60. Fig. 8-18 Rate of reaction Optimal temperature for enzyme of thermophilic (heat-tolerant) bacteria Optimal temperature for
- 61. Cofactors Cofactors are nonprotein enzyme helpers Cofactors may be inorganic (such as a metal in ionic
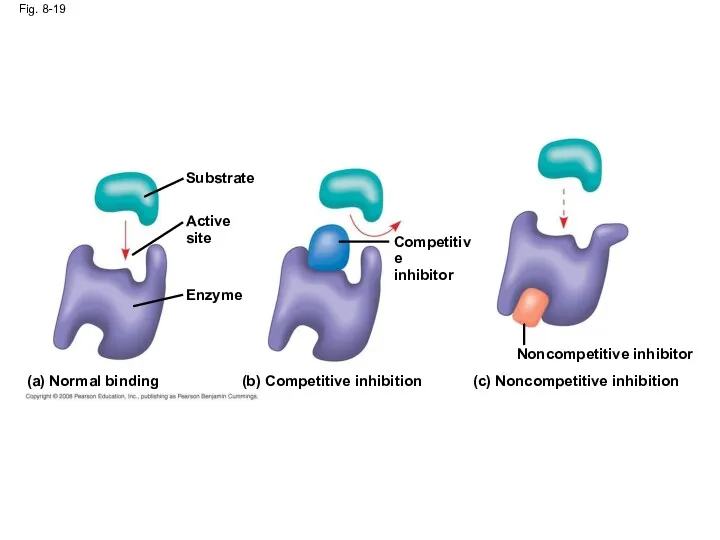
- 62. Enzyme Inhibitors Competitive inhibitors bind to the active site of an enzyme, competing with the substrate
- 63. Fig. 8-19 (a) Normal binding (c) Noncompetitive inhibition (b) Competitive inhibition Noncompetitive inhibitor Active site Competitive
- 64. Concept 8.5: Regulation of enzyme activity helps control metabolism Chemical chaos would result if a cell’s
- 65. Allosteric Regulation of Enzymes Allosteric regulation may either inhibit or stimulate an enzyme’s activity Allosteric regulation
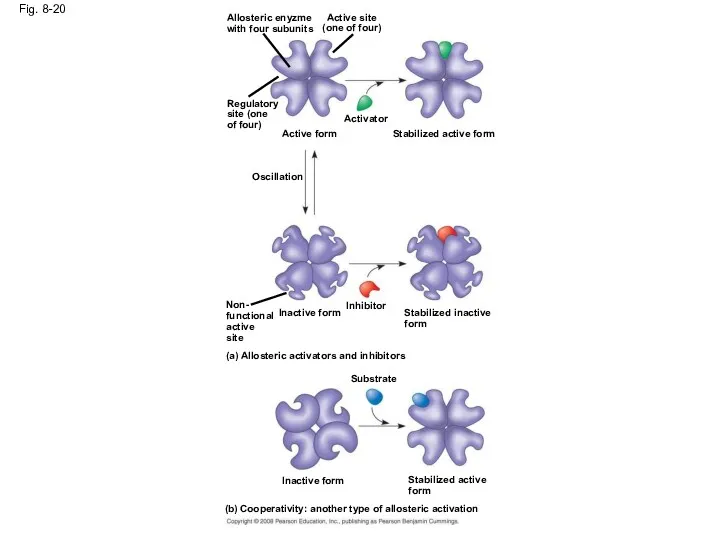
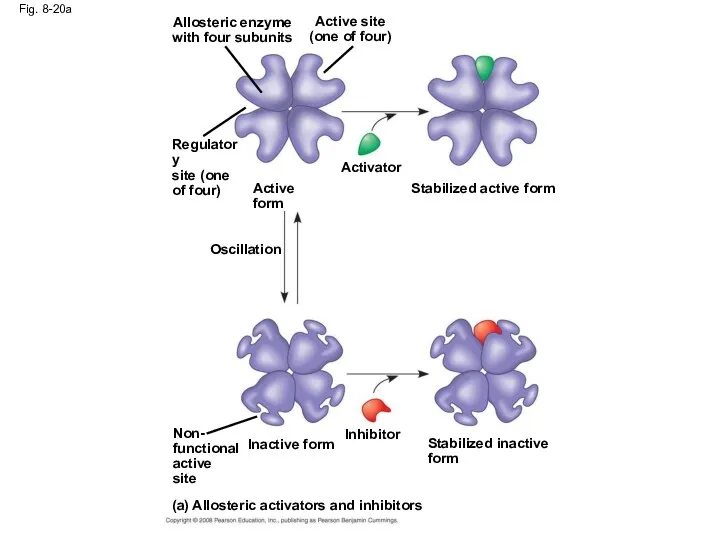
- 66. Allosteric Activation and Inhibition Most allosterically regulated enzymes are made from polypeptide subunits Each enzyme has
- 67. Fig. 8-20 Allosteric enyzme with four subunits Active site (one of four) Regulatory site (one of
- 68. Fig. 8-20a (a) Allosteric activators and inhibitors Inhibitor Non- functional active site Stabilized inactive form Inactive
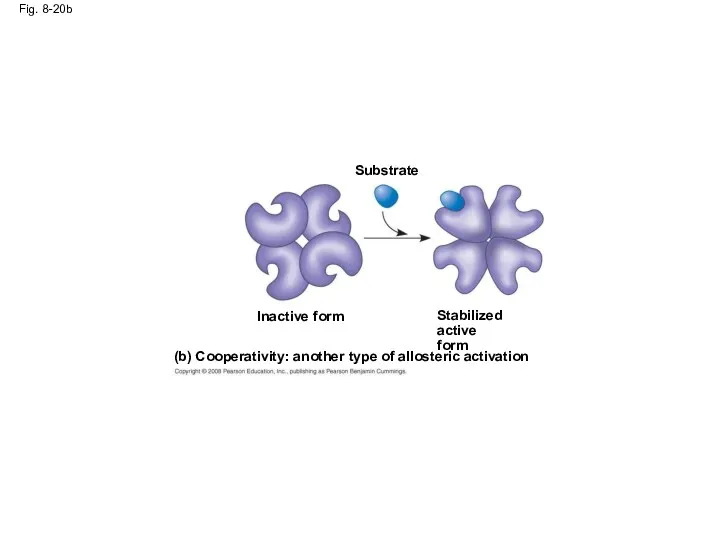
- 69. Cooperativity is a form of allosteric regulation that can amplify enzyme activity In cooperativity, binding by
- 70. Fig. 8-20b (b) Cooperativity: another type of allosteric activation Stabilized active form Substrate Inactive form
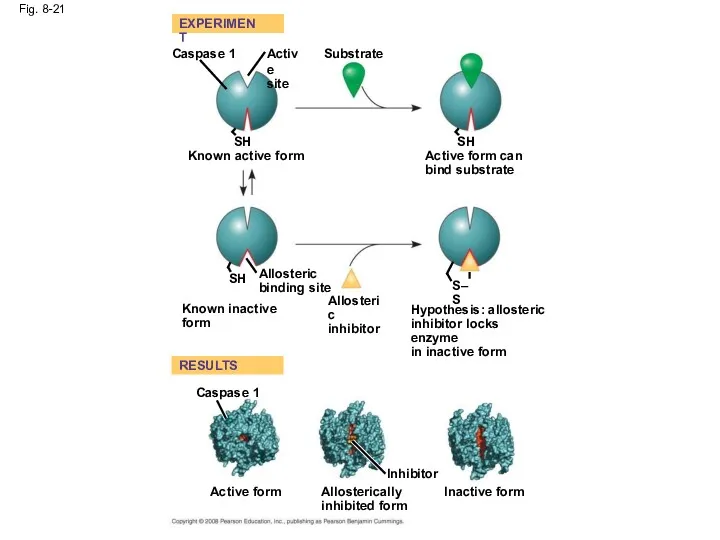
- 71. Identification of Allosteric Regulators Allosteric regulators are attractive drug candidates for enzyme regulation Inhibition of proteolytic
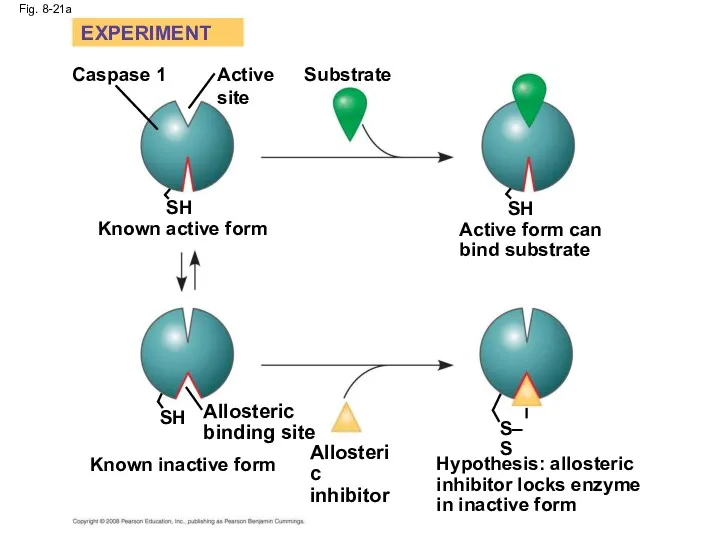
- 72. Fig. 8-21 RESULTS EXPERIMENT Caspase 1 Active site SH Known active form Substrate SH Active form
- 73. Fig. 8-21a SH Substrate Hypothesis: allosteric inhibitor locks enzyme in inactive form Active form can bind
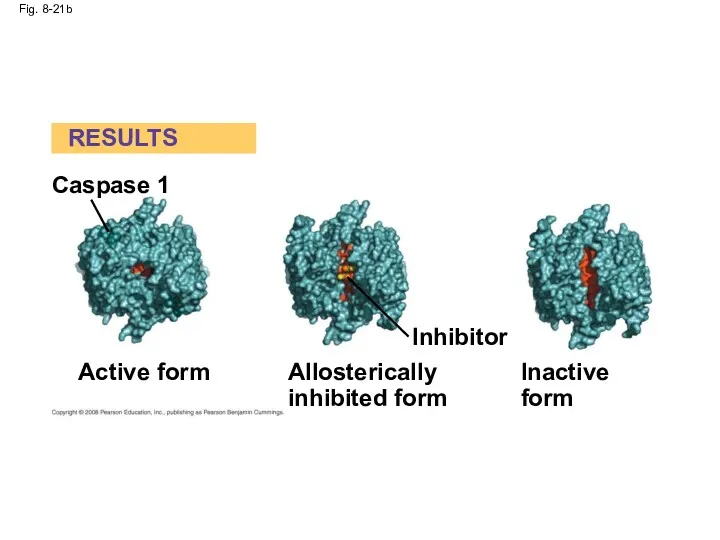
- 74. Fig. 8-21b Caspase 1 RESULTS Active form Inhibitor Allosterically inhibited form Inactive form
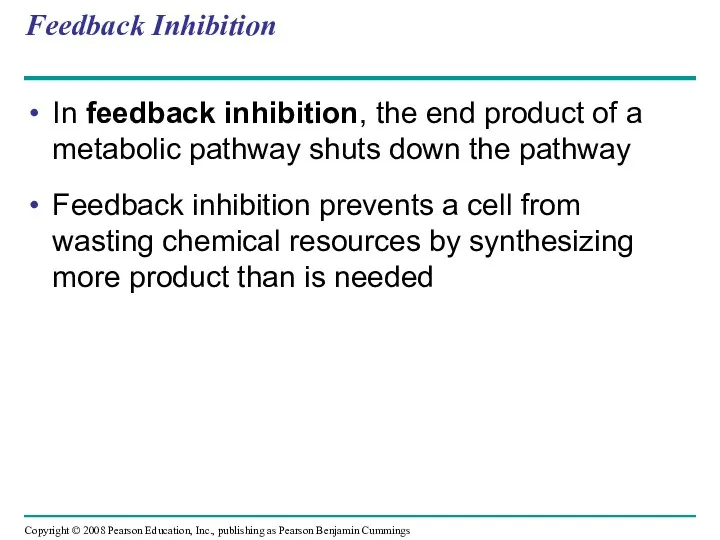
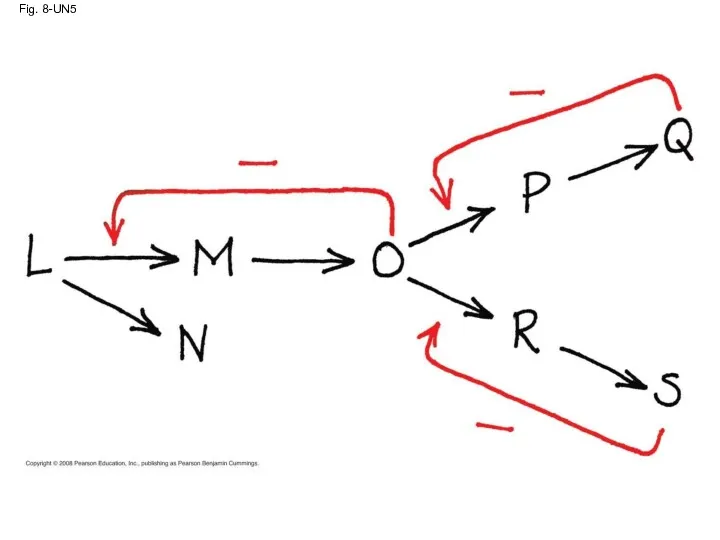
- 75. Feedback Inhibition In feedback inhibition, the end product of a metabolic pathway shuts down the pathway
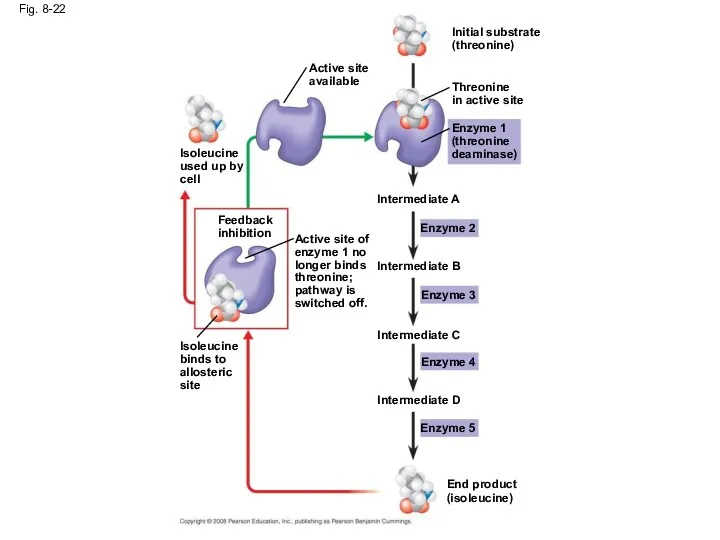
- 76. Fig. 8-22 Intermediate C Feedback inhibition Isoleucine used up by cell Enzyme 1 (threonine deaminase) End
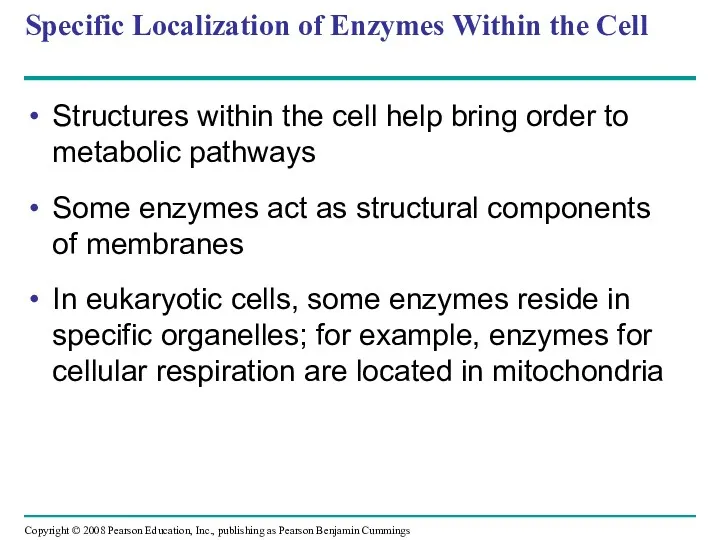
- 77. Specific Localization of Enzymes Within the Cell Structures within the cell help bring order to metabolic
- 78. Fig. 8-23 1 µm Mitochondria
- 79. Fig. 8-UN2 Progress of the reaction Products Reactants ∆G is unaffected by enzyme Course of reaction
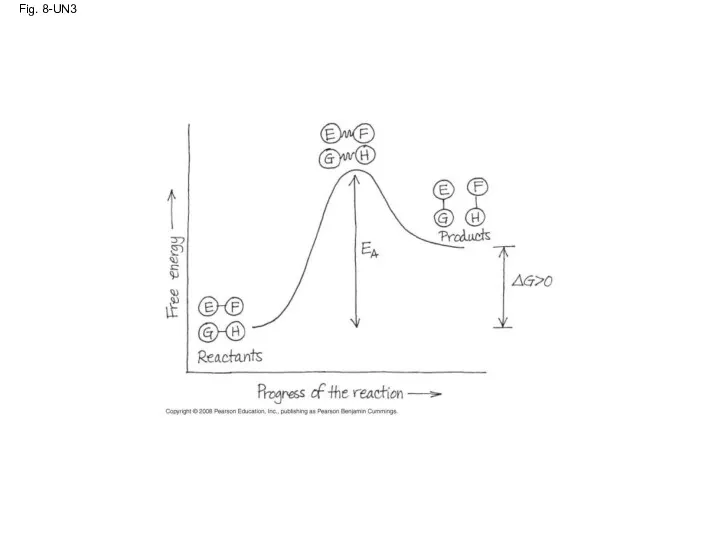
- 80. Fig. 8-UN3
- 81. Fig. 8-UN4
- 82. Fig. 8-UN5
- 83. You should now be able to: Distinguish between the following pairs of terms: catabolic and anabolic
- 85. Скачать презентацию








 Призентация Современные образовательные технологии
Призентация Современные образовательные технологии Простые поделки на основе ладошки
Простые поделки на основе ладошки Становление информационного общества
Становление информационного общества Биография Александра Дюма
Биография Александра Дюма Митоздың профазасы және метафазасы. Митозсимулдаушы фактор (МСФ) әрекеттері
Митоздың профазасы және метафазасы. Митозсимулдаушы фактор (МСФ) әрекеттері Соединение в художественном произведении двух реальностей – действительности и фантазии
Соединение в художественном произведении двух реальностей – действительности и фантазии Правила поведения в группе продленного дня презентация
Правила поведения в группе продленного дня презентация Влияние заполнителей на прочность бетона. Упругость бетона
Влияние заполнителей на прочность бетона. Упругость бетона Фундаменты. Схема устройства фундаментов
Фундаменты. Схема устройства фундаментов Аналитический отчет
Аналитический отчет Презентация Азбука ч.3 Диск
Презентация Азбука ч.3 Диск Тиснение по фольге - чеканка
Тиснение по фольге - чеканка Организация работ по диагностированию, техническому обслуживанию и ремонту ходовой части ВАЗ 2105
Организация работ по диагностированию, техническому обслуживанию и ремонту ходовой части ВАЗ 2105 Многоэтажные здания
Многоэтажные здания Афинский Акрополь. Греческая архитектура и скульптура
Афинский Акрополь. Греческая архитектура и скульптура Роль медицинской сестры в реабилитации после инсульта
Роль медицинской сестры в реабилитации после инсульта CRM для малого бизнеса. Работа с клиентами, управление продажами и рабочим временем
CRM для малого бизнеса. Работа с клиентами, управление продажами и рабочим временем Перитонит и сепсис
Перитонит и сепсис Ты сам мастер декоративно-прикладного искусства
Ты сам мастер декоративно-прикладного искусства ПТЭ, инструкции и безопасность движения
ПТЭ, инструкции и безопасность движения Конспект и презентация к уроку технологии Откуда берутся нитки
Конспект и презентация к уроку технологии Откуда берутся нитки Поисковая система Яндекс
Поисковая система Яндекс Чтение информации на этикетке упакованного товара и изучение его подлинности по штриховому коду
Чтение информации на этикетке упакованного товара и изучение его подлинности по штриховому коду ОАО Нэфис Косметикс-производитель товаров повседневного спроса
ОАО Нэфис Косметикс-производитель товаров повседневного спроса Общая характеристика металлов главных подгрупп I - III групп ПСХЭ Д.И.Менделеева
Общая характеристика металлов главных подгрупп I - III групп ПСХЭ Д.И.Менделеева Сетевое взаимодействие через сокеты
Сетевое взаимодействие через сокеты Работа с одарёнными детьми
Работа с одарёнными детьми Истина и ее критерии
Истина и ее критерии