Содержание
- 2. Serology Nucleic Acid Testing Personalized Lab Automation RBSS - Roche Blood Safety Solutions Designed to work
- 3. Serology Nucleic Acid Testing Personalized Lab Automation Serology Nucleic Acid Testing Personalized Lab Automation cobas s
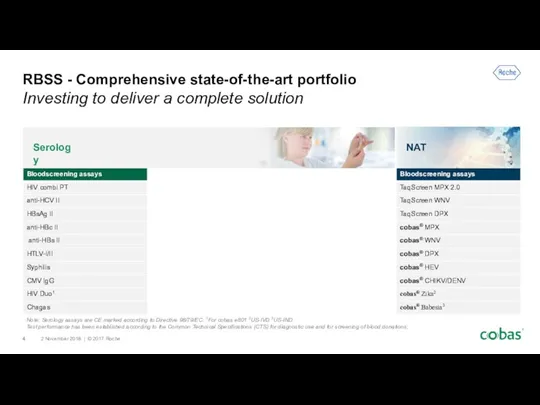
- 4. RBSS - Comprehensive state-of-the-art portfolio Investing to deliver a complete solution Note: Serology assays are CE
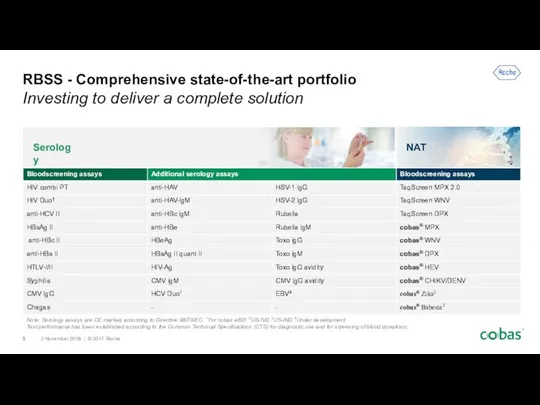
- 5. RBSS - Comprehensive state-of-the-art portfolio Investing to deliver a complete solution Note: Serology assays are CE
- 6. RBSS - Roche Blood Safety Solutions Safety is key Safety Reliability Efficiency
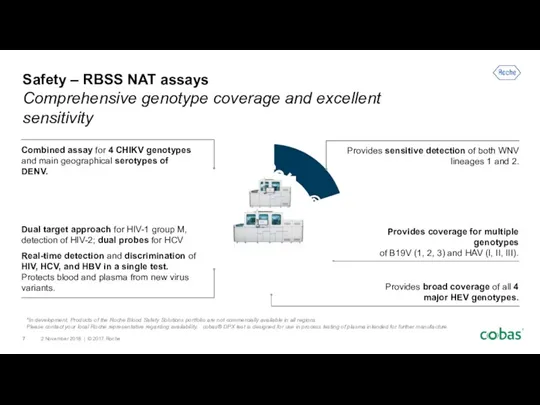
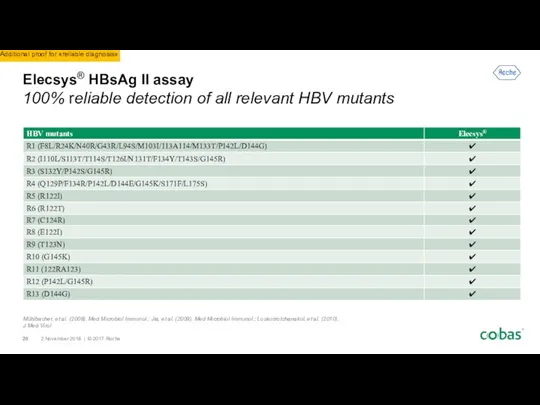
- 7. Provides coverage for multiple genotypes of B19V (1, 2, 3) and HAV (I, II, III). Provides
- 8. Safety – RBSS Serology assays Comprehensive genotype coverage and excellent sensitivity Sources: HTLV I/II Elecsys cobas
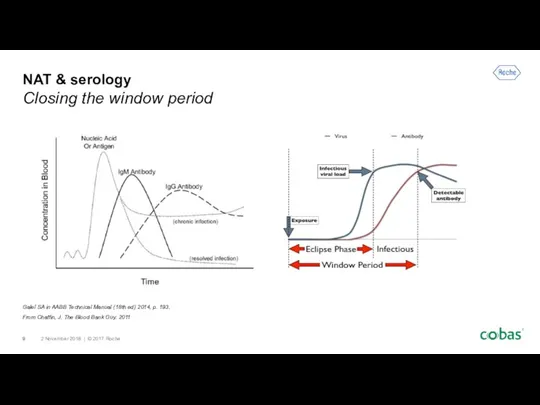
- 9. NAT & serology Closing the window period Galel SA in AABB Technical Manual (18th ed) 2014,
- 10. RBSS - Roche Blood Safety Solutions Supporting a consistent and dependable blood supply Safety Reliability Efficiency
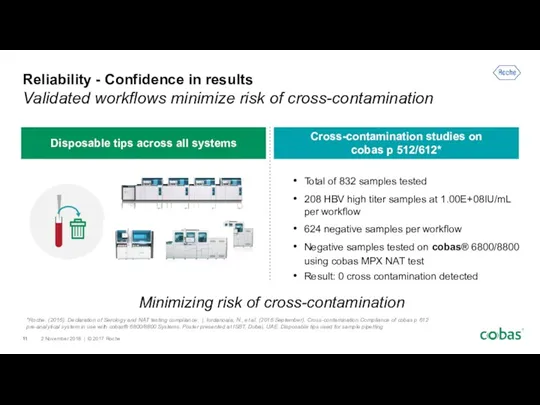
- 11. Reliability - Confidence in results Validated workflows minimize risk of cross-contamination Total of 832 samples tested
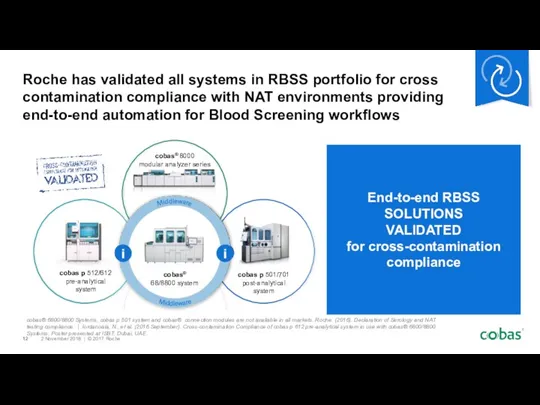
- 12. End-to-end RBSS SOLUTIONS VALIDATED for cross-contamination compliance Roche has validated all systems in RBSS portfolio for
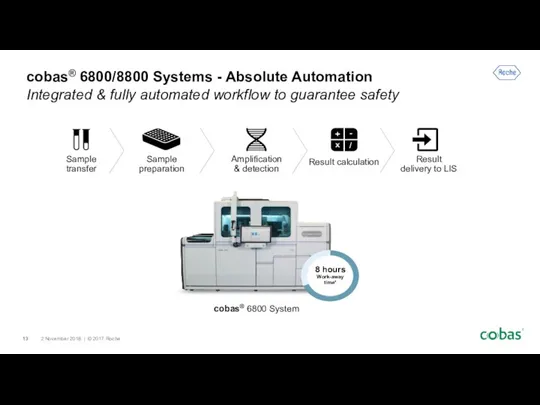
- 13. cobas® 6800/8800 Systems - Absolute Automation Integrated & fully automated workflow to guarantee safety
- 14. Fully Automated Contamination Prevention Ensures confidence in sample & result integrity Physical Chemical Photo of airlock
- 15. RBSS - Roche Blood Safety Solutions Realize efficiency gains Safety Reliability Efficiency
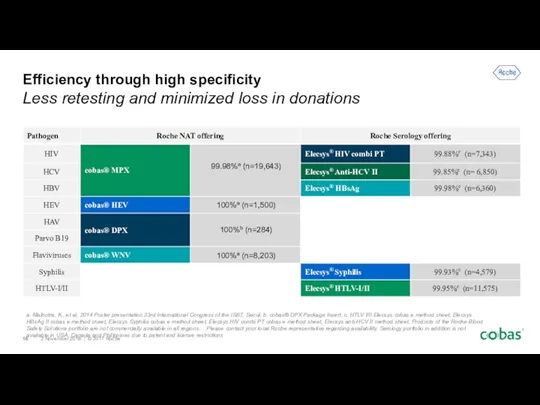
- 16. Efficiency through high specificity Less retesting and minimized loss in donations a. Malhotra, K., et al.
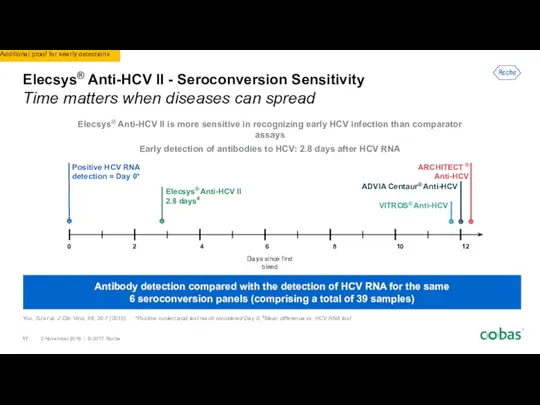
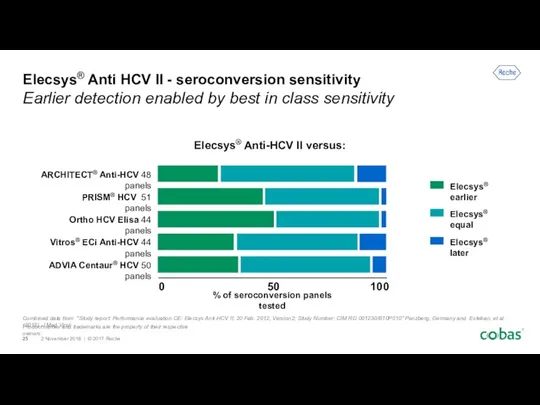
- 17. Elecsys® Anti-HCV II - Seroconversion Sensitivity Time matters when diseases can spread Antibody detection compared with
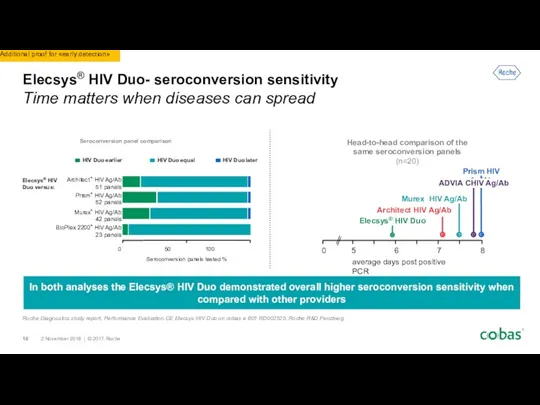
- 18. Elecsys® HIV Duo- seroconversion sensitivity Time matters when diseases can spread In both analyses the Elecsys®
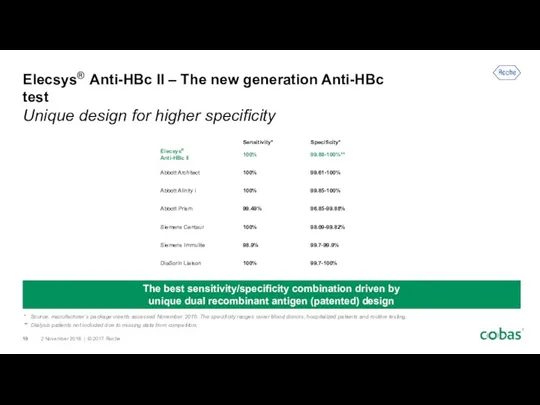
- 19. Elecsys® Anti-HBc II – The new generation Anti-HBc test Unique design for higher specificity The best
- 20. Mühlbacher, et al. (2008). Med Microbiol Immunol.; Jia, et al. (2009). Med Microbiol Immunol.; Louisirirotchanakul, et
- 21. RBSS - Roche Blood Safety Solutions Dedicated to blood screening since 1998 Safety Reliability Efficiency
- 22. Doing now what patients need next
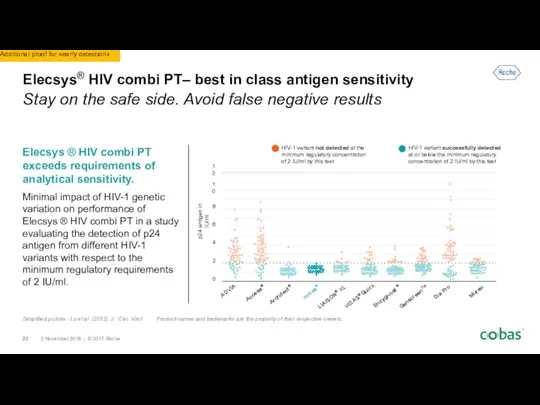
- 23. Elecsys® HIV combi PT– best in class antigen sensitivity Stay on the safe side. Avoid false
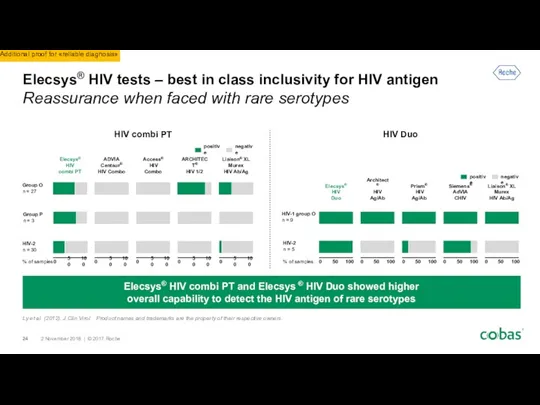
- 24. Elecsys® HIV tests – best in class inclusivity for HIV antigen Reassurance when faced with rare
- 25. Combined data from "Study report: Performance evaluation CE: Elecsys Anti-HCV II; 20 Feb. 2012, Version2; Study
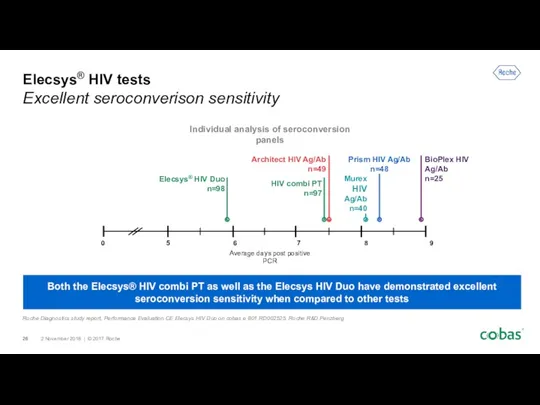
- 26. Elecsys® HIV tests Excellent seroconverison sensitivity Both the Elecsys® HIV combi PT as well as the
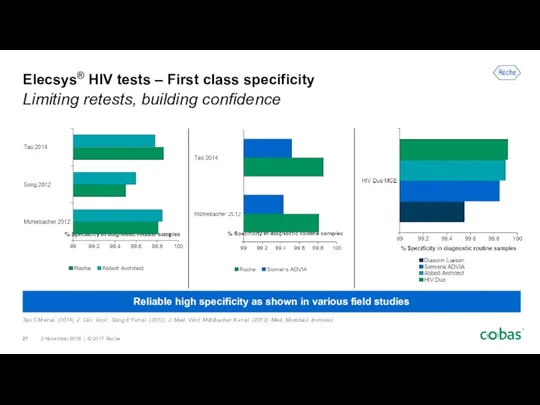
- 27. Reliable high specificity as shown in various field studies Elecsys® HIV tests – First class specificity
- 29. Скачать презентацию




 Небесный металл
Небесный металл Корпоративные финансы. Экономическое содержание и назначение корпоративных финансов
Корпоративные финансы. Экономическое содержание и назначение корпоративных финансов ДЕТИ ВОЙНЫ (презентация)
ДЕТИ ВОЙНЫ (презентация) SP MDT MANUAL
SP MDT MANUAL Интерактивная тематическая игра Что такое толерантность? (Поле чудес)
Интерактивная тематическая игра Что такое толерантность? (Поле чудес) Лабиринты 5-6 классы
Лабиринты 5-6 классы Водопровідні мережі. Режими водоспоживання. Витрати та напори в протипожежних водопроводах
Водопровідні мережі. Режими водоспоживання. Витрати та напори в протипожежних водопроводах Песенка про Китай
Песенка про Китай Ангелы
Ангелы Анализ программ нового поколения для подготовки детей к школе
Анализ программ нового поколения для подготовки детей к школе Один день из жизни учителя
Один день из жизни учителя Вредные привычки и их влияние на здоровье подростков
Вредные привычки и их влияние на здоровье подростков Викторина для детей от 6 – 7 лет
Викторина для детей от 6 – 7 лет Урал. Путешествие по России
Урал. Путешествие по России Карбонильные соединения и карбоновые кислоты. Лекция № 4
Карбонильные соединения и карбоновые кислоты. Лекция № 4 Звуковой и слоговой анализ при автоматизации звука Ль
Звуковой и слоговой анализ при автоматизации звука Ль Духовно-нравственное воспитание
Духовно-нравственное воспитание Урок мира и добра ( классный час )
Урок мира и добра ( классный час ) Автокөліктерді диагностикалау
Автокөліктерді диагностикалау Образы русской природы. С.Я. Маршак Гроза днём, В лесу над росистой поляной
Образы русской природы. С.Я. Маршак Гроза днём, В лесу над росистой поляной Моделирование алгоритмов вейвлет-преобразования. Вейвлет-фильтры и их характеристики
Моделирование алгоритмов вейвлет-преобразования. Вейвлет-фильтры и их характеристики Наряд для семейного обеда. (Технология, 6 класс)
Наряд для семейного обеда. (Технология, 6 класс) My idol - hockey club AK BARS
My idol - hockey club AK BARS Транспорт и его виды
Транспорт и его виды Приложение 1 к постановлению Правительства Республики Казахстан
Приложение 1 к постановлению Правительства Республики Казахстан Физиологическая желтуха новорожденных
Физиологическая желтуха новорожденных Интегрированное занятие: Как называют Деда Мороза в разных странах мира? Вторая младшая группа. Воспитатель: Котюжанская Ольга Игоревна.
Интегрированное занятие: Как называют Деда Мороза в разных странах мира? Вторая младшая группа. Воспитатель: Котюжанская Ольга Игоревна. Принципы этиопатогенетической терапии фарингитов
Принципы этиопатогенетической терапии фарингитов