Содержание
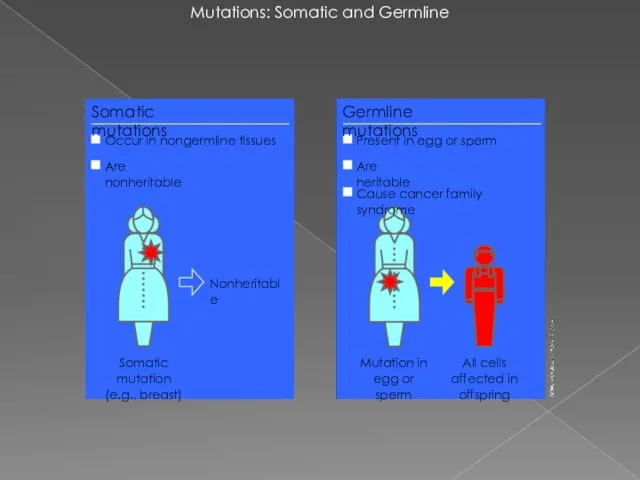
- 2. Mutations: Somatic and Germline Mutation in egg or sperm Nonheritable Somatic mutations Occur in nongermline tissues
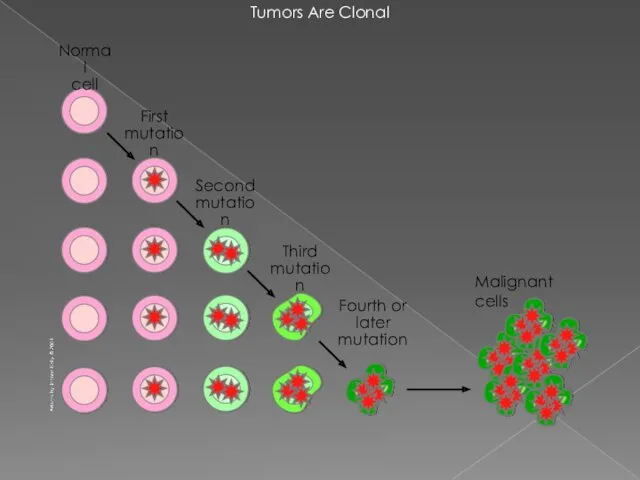
- 3. Tumors Are Clonal Malignant cells
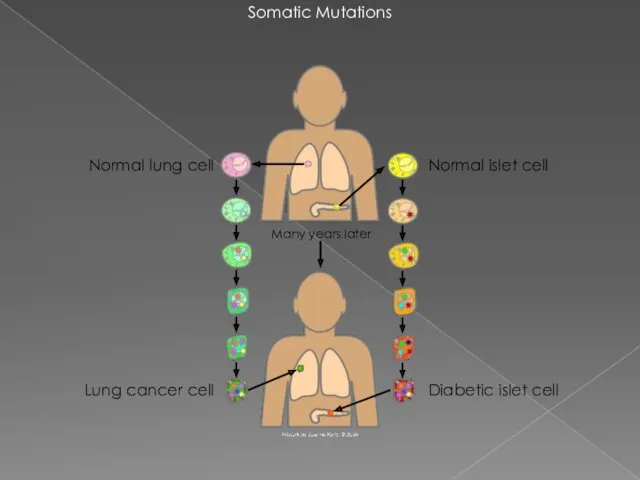
- 4. Somatic Mutations Diabetic islet cell Normal islet cell Normal lung cell Lung cancer cell Many years
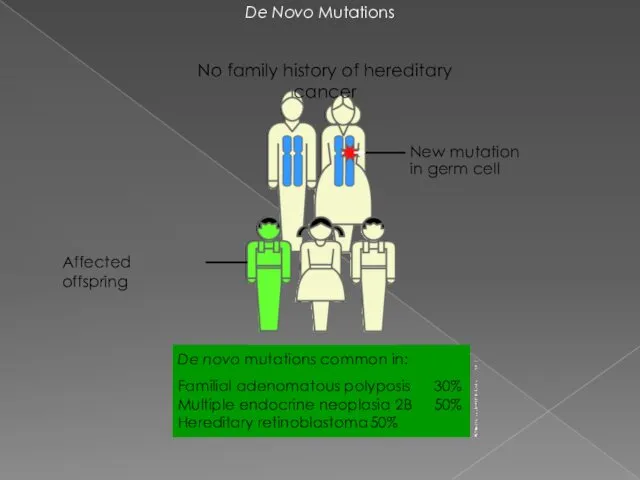
- 5. De Novo Mutations New mutation in germ cell No family history of hereditary cancer De novo
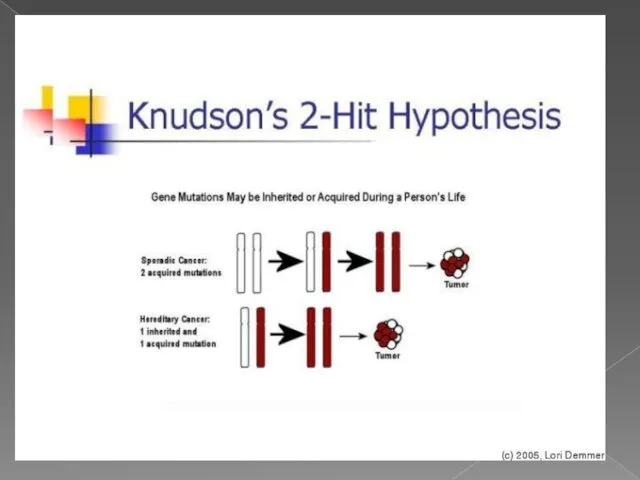
- 6. теория двойного удара или двойной мутации В 1971 году Альфред Кнудсон предложил гипотезу, известную сейчас как
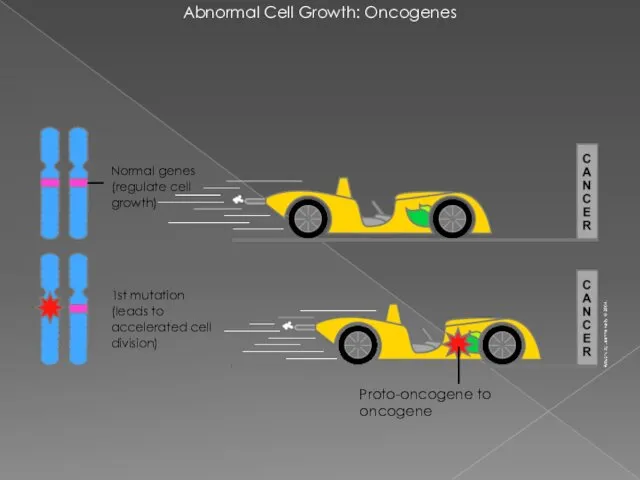
- 9. ОНКОГЕН — это ген, продукт которого может стимулировать образование злокачественной опухоли. Мутации, вызывающие активацию онкогенов, повышают
- 10. Протоонкоген — это обычный ген, который может стать онкогеном из-за мутаций или повышения экспрессии. Многие протоонкогены
- 11. Протоонкоген может стать онкогеном путем относительно незначительной модификации его естественной функции. три основных пути активации: 1.
- 12. Abnormal Cell Growth: Oncogenes Proto-oncogene to oncogene 1st mutation (leads to accelerated cell division) Normal genes
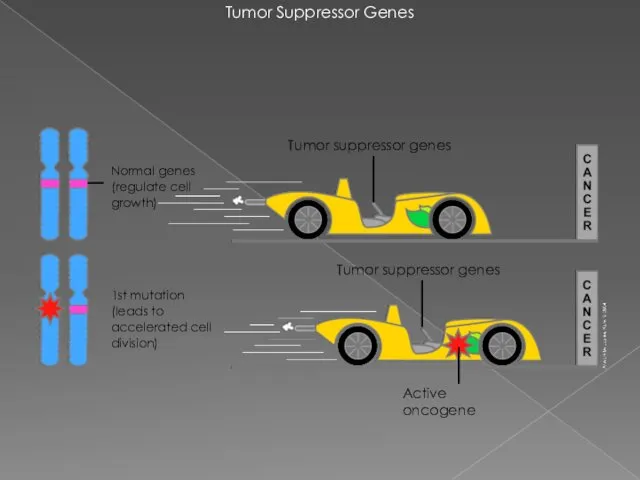
- 13. Tumor Suppressor Genes 1st mutation (leads to accelerated cell division) Normal genes (regulate cell growth) Tumor
- 14. Mutations in Tumor Suppressor Genes 1st mutation (susceptible carrier) Active oncogene No brakes! Active oncogene Normal
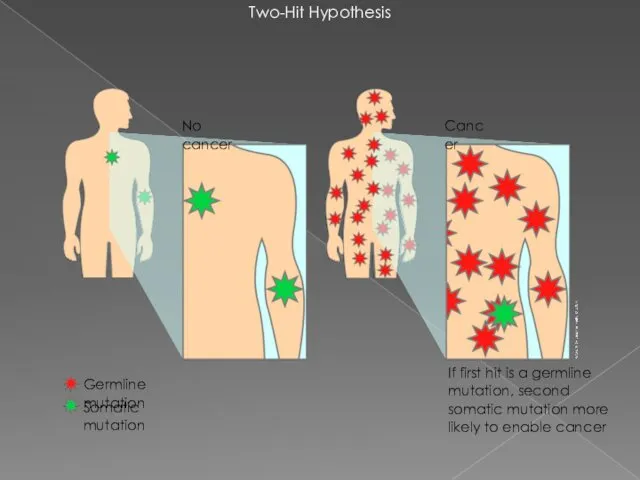
- 15. Two-Hit Hypothesis If first hit is a germline mutation, second somatic mutation more likely to enable
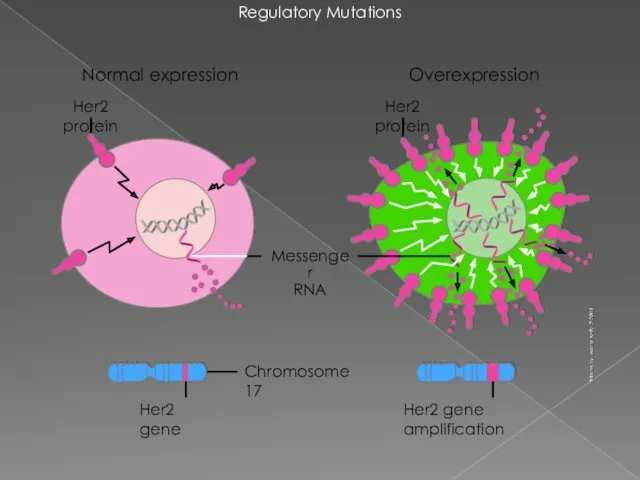
- 16. Regulatory Mutations Chromosome 17 Messenger RNA Her2 gene Her2 gene amplification Overexpression Her2 protein Her2 protein
- 17. Translocation of Bcr-Abl Genes Fusion protein with tyrosine kinase activity (q+) Ph (22q–) bcr-abl abl bcr
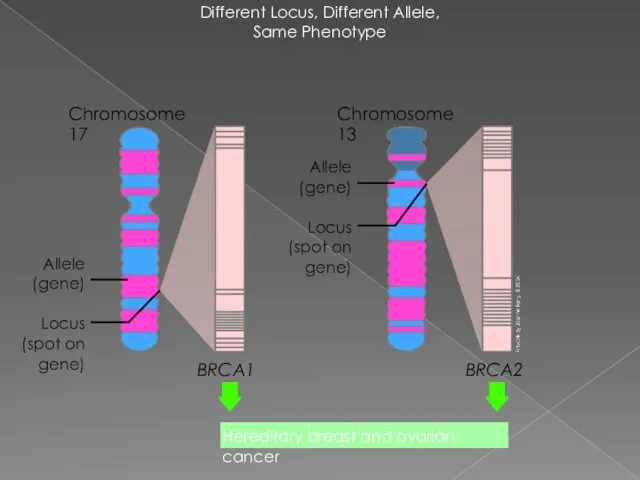
- 18. Different Locus, Different Allele, Same Phenotype Chromosome 17 BRCA1 BRCA2 Locus (spot on gene) Allele (gene)
- 19. Founder Effect in Ashkenazi Jewish Population An estimated 1 in 40 Ashkenazi Jews carries a BRCA1
- 20. Mutations in Cancer Susceptibility Genes: BRCA1 Nonsense/Frameshift Missense Splice-site Protein has role in genomic stability ~500
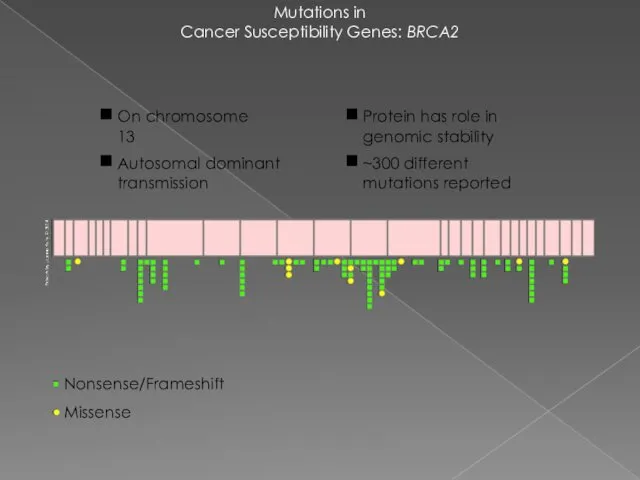
- 21. Mutations in Cancer Susceptibility Genes: BRCA2 Nonsense/Frameshift Missense Protein has role in genomic stability ~300 different
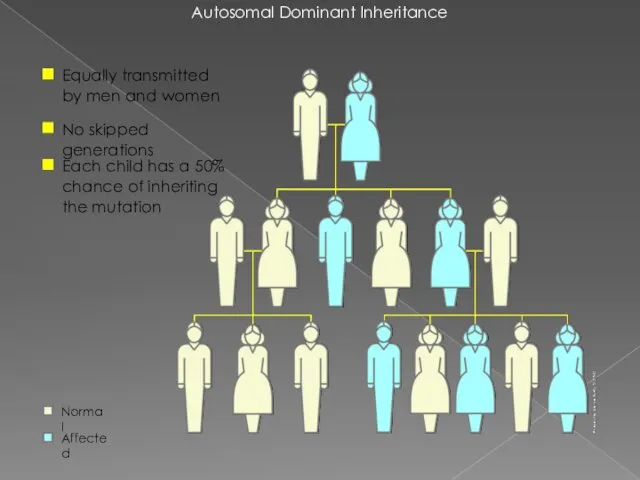
- 22. Autosomal Dominant Inheritance Equally transmitted by men and women No skipped generations Each child has a
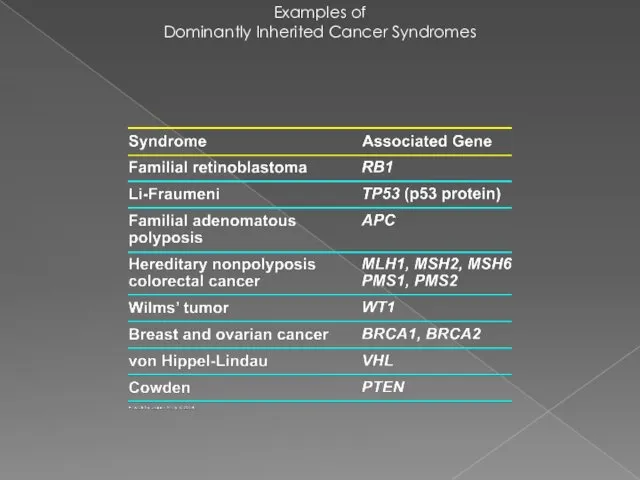
- 23. Examples of Dominantly Inherited Cancer Syndromes
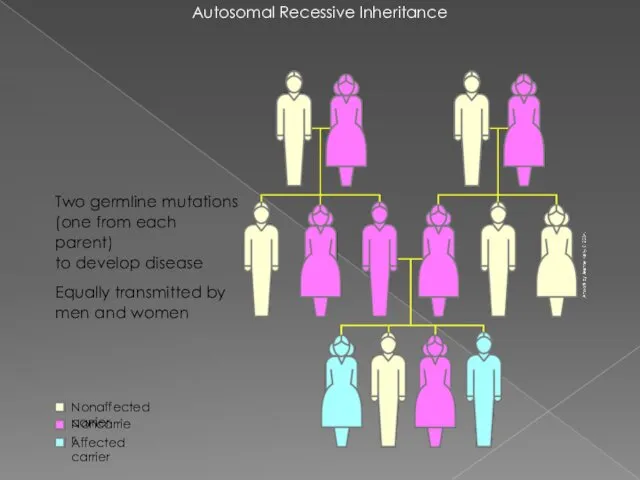
- 24. Autosomal Recessive Inheritance Two germline mutations (one from each parent) to develop disease Equally transmitted by
- 25. Some Recessively Inherited Cancer Syndromes
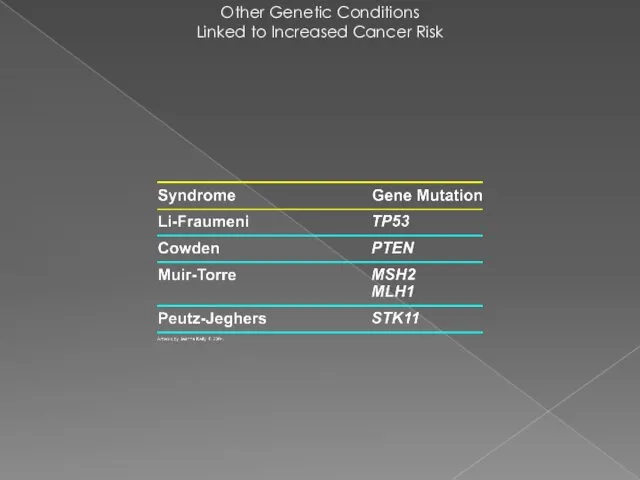
- 26. Other Genetic Conditions Linked to Increased Cancer Risk
- 27. Repair Failure Cancer Aging Inborn disease (Transient) cell cycle arrest Apoptosis (cell death) Nucleotide-excision repair (NER)
- 28. Cancer Susceptibility: Much Still Unknown
- 29. How do people know if they should consider genetic testing for BRCA1 and BRCA2 mutations? For
- 30. Li-Fraumeni Syndrome Li-Fraumeni Syndrome (LFS) was first described in 1969 by Drs. Frederick Li and Joseph
- 31. Classic Li-Fraumeni Syndrome (LFS): Three features must be present in a family to fit the classic
- 32. Li-Fraumeni-Like Syndrome (LFL): A person with any childhood cancer or sarcoma, brain tumor, or adrenal cortical
- 33. What Causes LFS? Changes in a “tumor suppressor” gene called “TP53” were discovered in 1990 as
- 34. Risk of Cancer in Patients with LFS The lifetime risk of cancer – all types combined
- 35. For now, in persons with a TP53 gene mutation, we can try to find cancers as
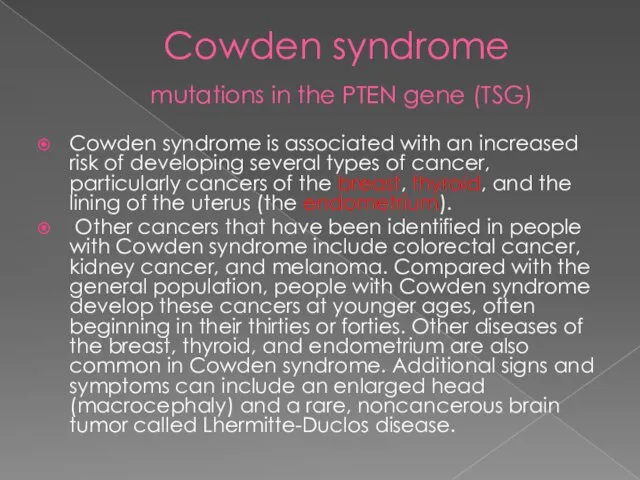
- 36. Cowden syndrome mutations in the PTEN gene Cowden syndrome is a disorder characterized by multiple noncancerous,
- 37. Cowden syndrome mutations in the PTEN gene (TSG) Cowden syndrome is associated with an increased risk
- 38. Что такое ОНКОГЕН ? 1.ген, стимулирующий образование опухоли 2. гены, предохраняющие клетки от ракового перерождения 3.
- 40. Скачать презентацию





















 Формирование учебных универсальных действий (УУД) во внеурочной деятельности учащихся
Формирование учебных универсальных действий (УУД) во внеурочной деятельности учащихся Индивидуальный проект Государственное устройство Древней Спарты
Индивидуальный проект Государственное устройство Древней Спарты Мои проекты
Мои проекты Эмиссия. Выпуск денег в хозяйственный оборот
Эмиссия. Выпуск денег в хозяйственный оборот Роль логистики в организации
Роль логистики в организации Витебская область
Витебская область Безопасность в сети интернет
Безопасность в сети интернет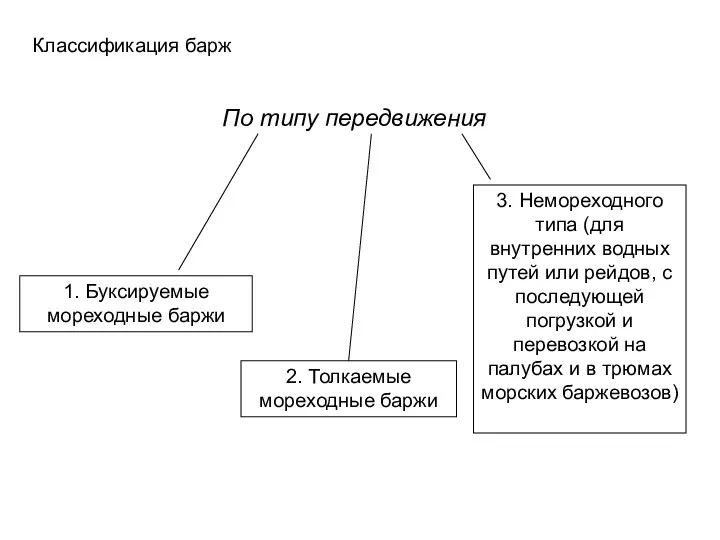 Классификация барж по типу передвижения
Классификация барж по типу передвижения Картотека зимние подвижные игры
Картотека зимние подвижные игры Конструкция скважины
Конструкция скважины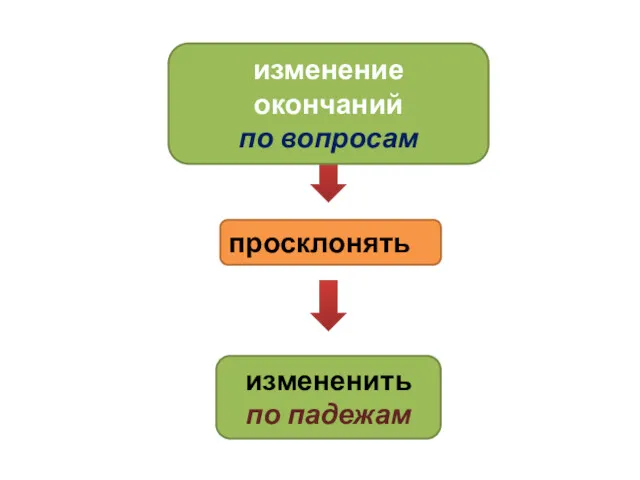 как определить падеж сущ-х
как определить падеж сущ-х Шаблонирование насосно - компрессорных труб (НКТ)
Шаблонирование насосно - компрессорных труб (НКТ) Лексика и фразеология
Лексика и фразеология Общие начала назначения наказания. УКРФ
Общие начала назначения наказания. УКРФ Прогнозирование течения эпилепсии на основе DFA анализа ЭЭГ
Прогнозирование течения эпилепсии на основе DFA анализа ЭЭГ Ты воспитатель, а это значит...
Ты воспитатель, а это значит... Кариес зубов у детей. Лечение
Кариес зубов у детей. Лечение Кейнсианская модель макроэкономического равновесия. (Тема 3)
Кейнсианская модель макроэкономического равновесия. (Тема 3) Франция. Экономическое развитие Франции. Политическое развитие Франции
Франция. Экономическое развитие Франции. Политическое развитие Франции Машины для разделения неоднородных систем
Машины для разделения неоднородных систем Медиакит радио GOLDSTAR
Медиакит радио GOLDSTAR Мировое хозяйство
Мировое хозяйство Компрессор ПК - 5,25 Тепловоза ТГМ-6
Компрессор ПК - 5,25 Тепловоза ТГМ-6 Презентация Наша Масленица
Презентация Наша Масленица Становление народного образования
Становление народного образования Отчёт о прохождении производственной практики
Отчёт о прохождении производственной практики Основные инициативы в области КСО и устойчивого развития. Отчетность компании в области КСО. Аудит отчетности
Основные инициативы в области КСО и устойчивого развития. Отчетность компании в области КСО. Аудит отчетности Всероссийский физкультурно-спортивный комплекс Готов к труду и обороне
Всероссийский физкультурно-спортивный комплекс Готов к труду и обороне