Содержание
- 2. Case Scenario Jane J. is a 22-year-old woman who was admitted to Community Hospital on May
- 3. Case Scenario “continued” Her hemoglobin was 6.3 g/dL (it had been 13.4 g/dL on a previous
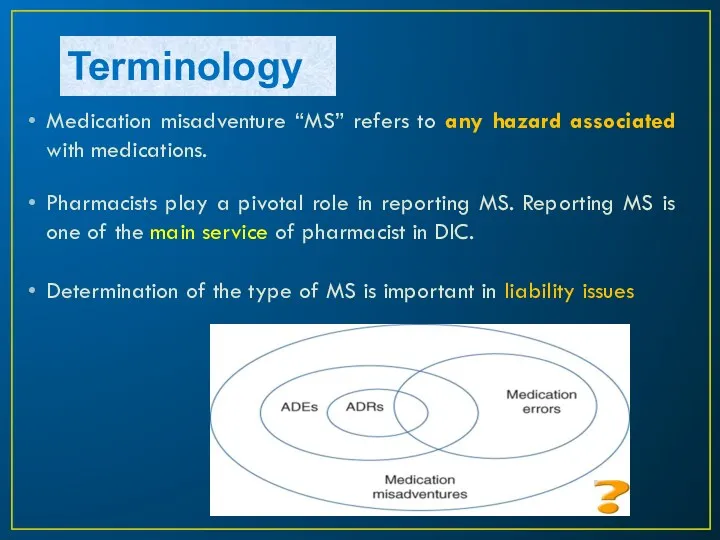
- 4. Terminology Medication misadventure “MS” refers to any hazard associated with medications. Pharmacists play a pivotal role
- 5. Terminology All adverse drug events (ADEs), adverse drug reactions (ADRs), and medication errors fall under the
- 6. Adverse Drug Reactions WHO defines an ADR as “any unintended response to a medicine which occurs
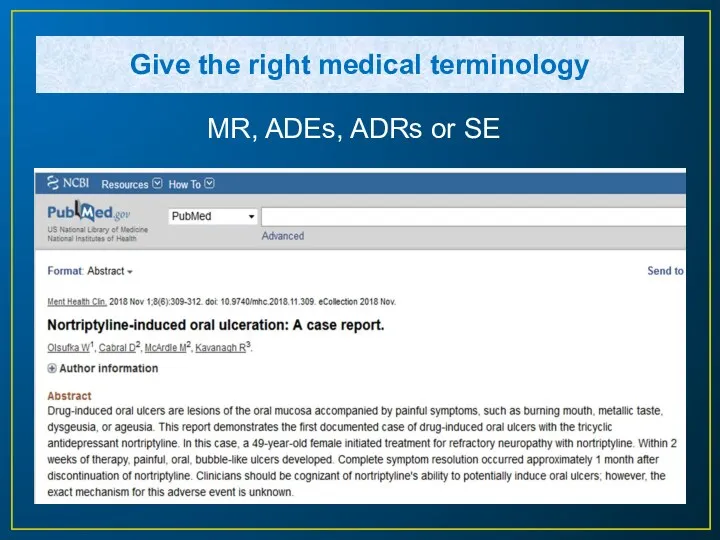
- 7. Give the right medical terminology MR, ADEs, ADRs or SE
- 8. DIC Pharmacist role & ADRs 1-updated with recent ADRs of drug in clinical practice & increase
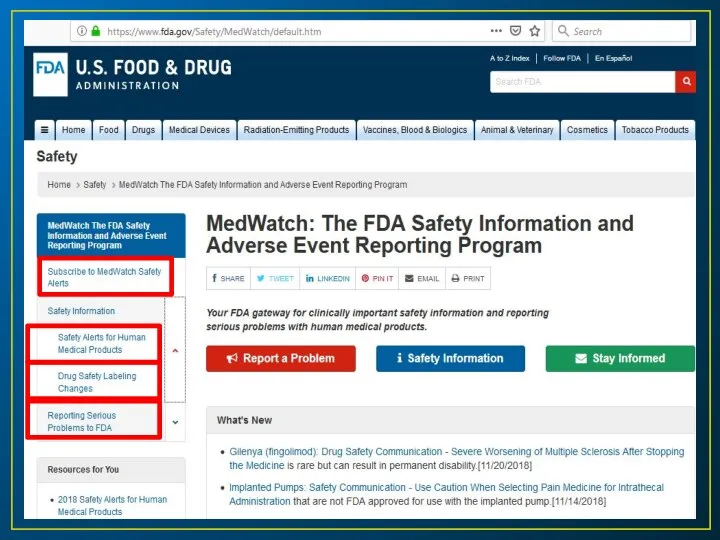
- 9. Resources for updates in ADRs The FDA Web (http://www.fda.gov/Safety/Medwatch). This online provide FDA’s latest safety alerts
- 11. Importance of Reporting ADRs 1- Postmarketing Surveillance of ADRs using Well-designed programs makes it possible to
- 12. Causality of ADRs To detect ADR, determine the causality “the probability that a particular drug causes
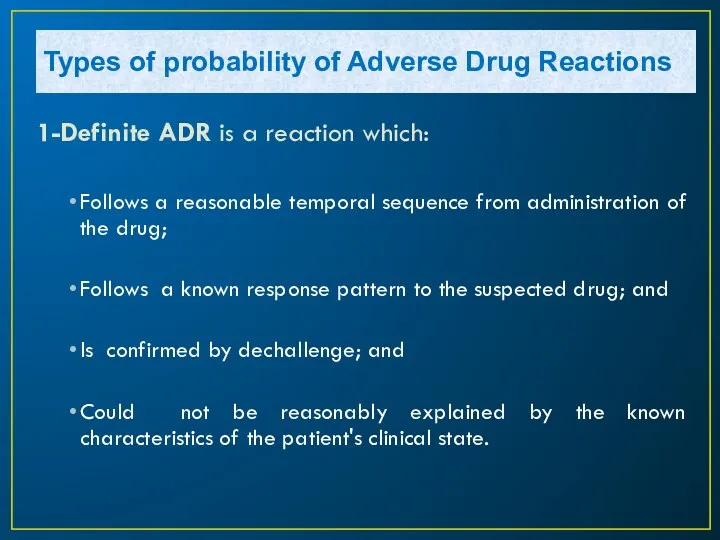
- 13. 1-Definite ADR is a reaction which: Follows a reasonable temporal sequence from administration of the drug;
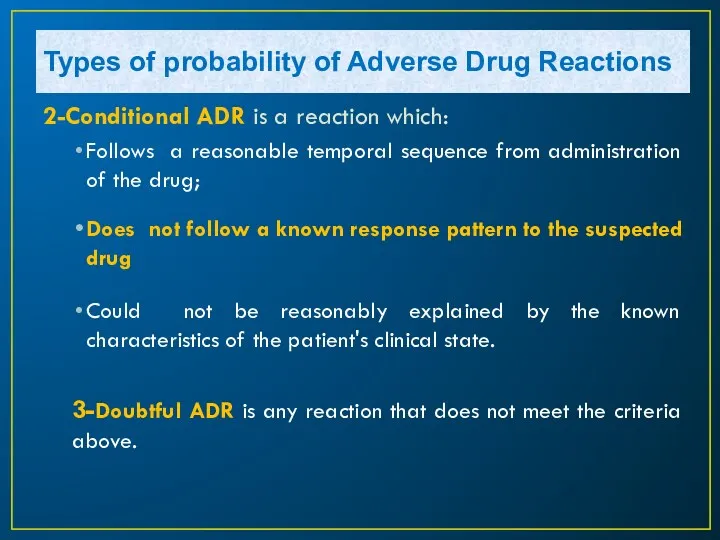
- 14. 2-Conditional ADR is a reaction which: Follows a reasonable temporal sequence from administration of the drug;
- 15. MedWatch program In June 1993, the FDA developed a new program called MedWatch. The current MedWatch
- 16. MedWatch Once submitted through the MedWatch system, ADR reports are received by a unit of the
- 20. Good Vigilance Practice (GVP) WHO stated for any company to be qualified for drug manufacturing and
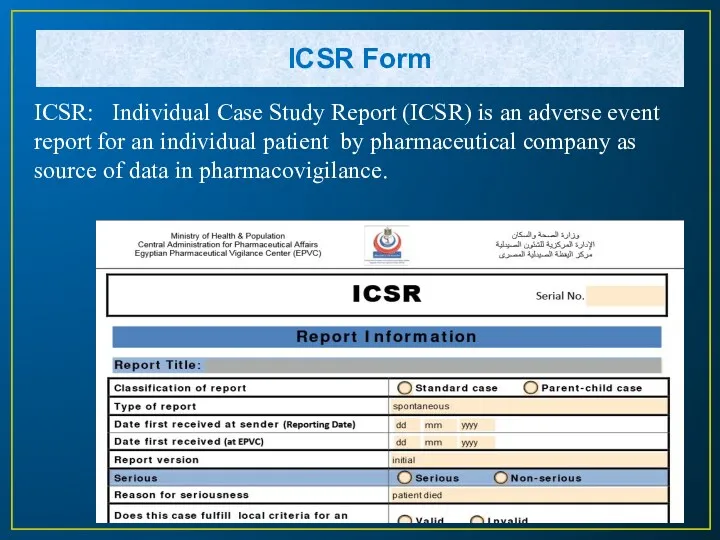
- 21. ICSR Form ICSR: Individual Case Study Report (ICSR) is an adverse event report for an individual
- 22. Pharmacovigilance Pharmacovigilance is the science and activities relating to the detection, assessment, handling and prevention of
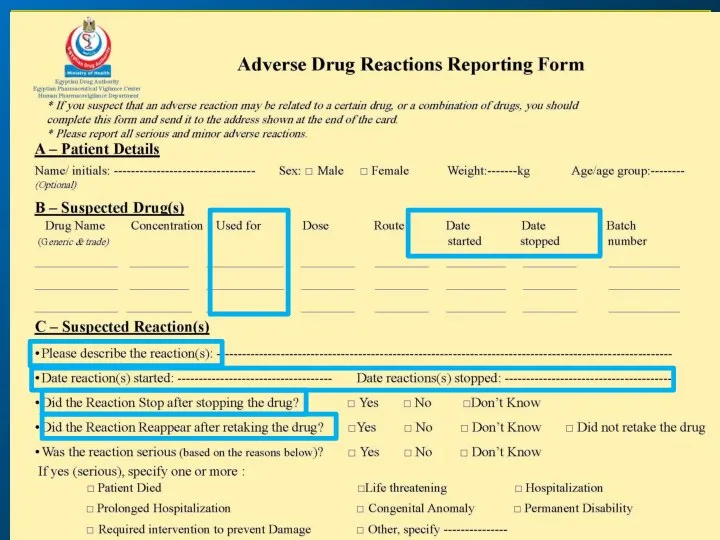
- 23. yellow card a unified form used to facilitate the reporting ADRs. The EPVC adapted this from
- 24. How to obtain the reporting form A web based dynamic reporting module is available at EPVC
- 27. Steps for Implementing a Program “ASHP Guidelines for P&TC” Develop definitions for ADRs and its seriousness
- 28. Report this ADRs to Yellow Card Jane J. is a 22-year-old woman who was admitted to
- 30. Скачать презентацию



















 Противоаварийная автоматика
Противоаварийная автоматика Презентация к уроку по географии (7 класс) на тему: Номенклатура Африки
Презентация к уроку по географии (7 класс) на тему: Номенклатура Африки Золотая цепь святости в культуре родного Подмосковья
Золотая цепь святости в культуре родного Подмосковья Тема урока: Раскрытие скобок (5 класс)
Тема урока: Раскрытие скобок (5 класс) Интерактивные возможности PowerPoint
Интерактивные возможности PowerPoint Пассивное использование солнечной энергии
Пассивное использование солнечной энергии Легенды и мифы Китая
Легенды и мифы Китая Обучение грамоте детей с речевыми нарушениями (1-я часть)
Обучение грамоте детей с речевыми нарушениями (1-я часть) Менингококкты инфекция
Менингококкты инфекция Методическая разработка ИГРЫ И СКАЗКИ НАРОДОВ РОССИИ
Методическая разработка ИГРЫ И СКАЗКИ НАРОДОВ РОССИИ Долгосрочная стратегия угледобычи ПСП Шахта Днепровская ПАО ДТЭК Павлоградуголь на период 2011 - 2030 годы
Долгосрочная стратегия угледобычи ПСП Шахта Днепровская ПАО ДТЭК Павлоградуголь на период 2011 - 2030 годы Система сбалансированных показателей. Показатели стратегических финансовых направлений
Система сбалансированных показателей. Показатели стратегических финансовых направлений 17227-integrirovannyj-urok-muzyki-i-okruzhayushchego-mira-po-teme-ty-i-tvoi-druzya.pptx
17227-integrirovannyj-urok-muzyki-i-okruzhayushchego-mira-po-teme-ty-i-tvoi-druzya.pptx В стране дорожных знаков
В стране дорожных знаков Здоровой питание
Здоровой питание Основні засади влади, керівництва та лідерства
Основні засади влади, керівництва та лідерства Проект на тему: О ЧЁМ МОЖЕТ РАССКАЗАТЬ БИБЛИОТЕКА
Проект на тему: О ЧЁМ МОЖЕТ РАССКАЗАТЬ БИБЛИОТЕКА Содержание основных структурных элементов организации расследования
Содержание основных структурных элементов организации расследования Опытно-эксперементальная деятельность по теме: Вода
Опытно-эксперементальная деятельность по теме: Вода Мотивационные структуры молодых ученых и разработчиков
Мотивационные структуры молодых ученых и разработчиков Молекулярно-генетический уровень организации живого
Молекулярно-генетический уровень организации живого Цифровые системы связи
Цифровые системы связи Пневмонии у детей
Пневмонии у детей Изменения в культуре и быте в первой четверти XVIII века
Изменения в культуре и быте в первой четверти XVIII века Работа с родителями!
Работа с родителями! Люди и коты
Люди и коты презентация на тему Игрушки Диск
презентация на тему Игрушки Диск Возможности использования учебного пособия Моя будущая профессия. Тесты по профессиональной ориентации школьников
Возможности использования учебного пособия Моя будущая профессия. Тесты по профессиональной ориентации школьников