Содержание
- 2. Outline General Introduction Materials and methods Results and Discussion Conclusion
- 3. Introduction What is happening in cement production nowadays? What kind problems cement industry face with? Portland
- 4. Introduction Why calcium sulphoaluminate cement? CSA have attracted the attention of scientists, as well as of
- 5. Introduction Hydration of CSA cement The hydration of cement is in simple terms of dissolution/precipitation process
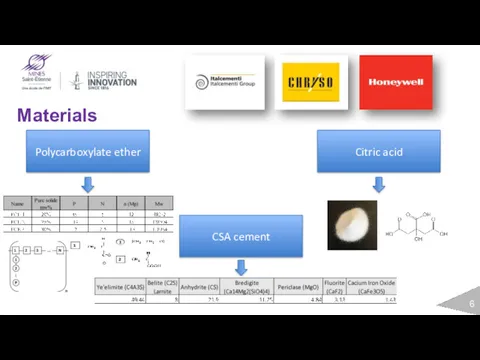
- 6. Materials CSA cement Polycarboxylate ether Citric acid
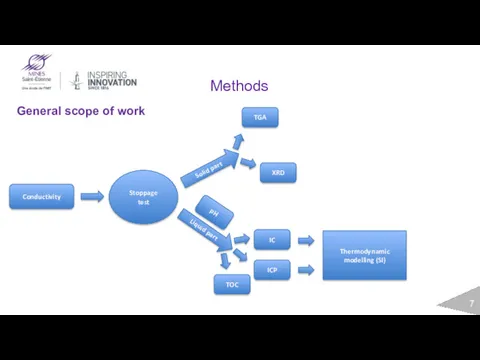
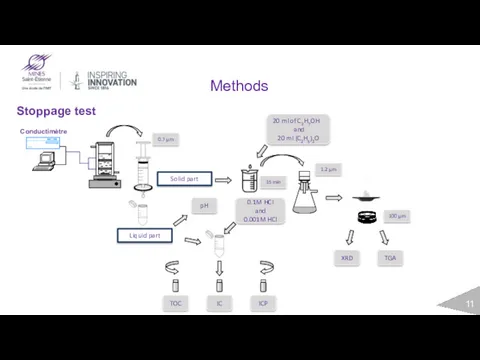
- 7. General scope of work Conductivity Stoppage test Solid part Liquid part ICP TOC IC TGA XRD
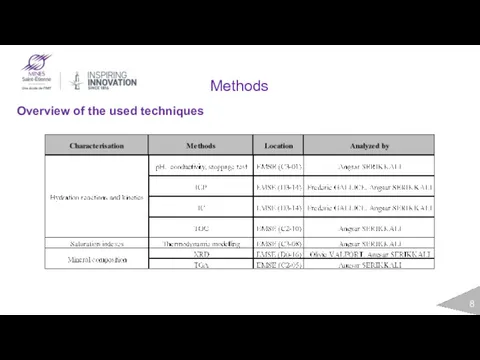
- 8. Methods Overview of the used techniques
- 9. Methods Conductivity V=1L Double walled and water-jacketed reactor T=24.6 ℃±0.2 270-350 r/min Experiment time is around
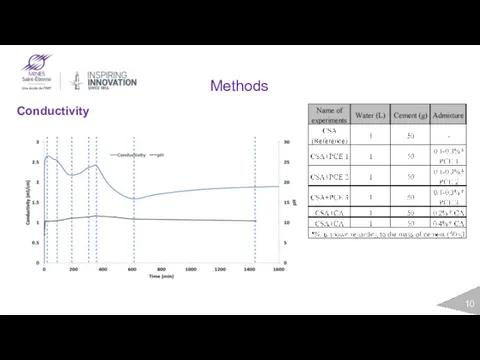
- 10. Methods Conductivity
- 11. Methods Stoppage test Liquid part Solid part TOC IC ICP TGA XRD 20 ml of C3H7OH
- 12. Methods Solid part and liquid part methods X-ray diffractometer (BRUKER D8-A25) X-ray tube (Cu radiation) X-ray
- 13. Methods Solid part and liquid part methods Instrument: V=8 ml with x10 dilution by 0.1M HCl
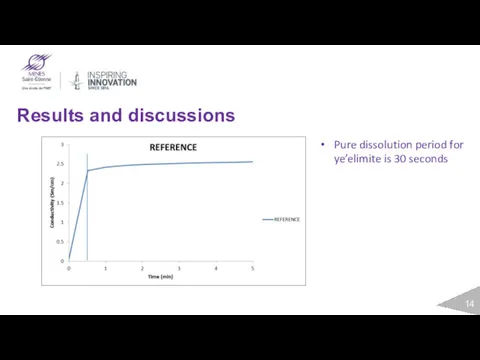
- 14. Results and discussions Pure dissolution period for ye’elimite is 30 seconds
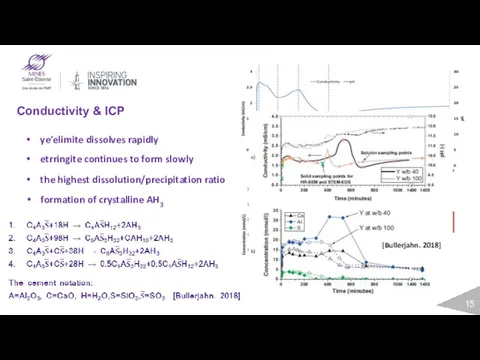
- 15. Conductivity & ICP ye’elimite dissolves rapidly etrringite continues to form slowly the highest dissolution/precipitation ratio formation
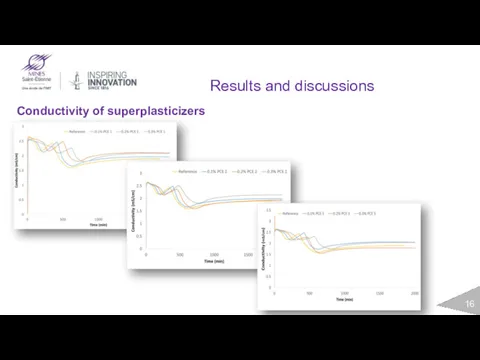
- 16. Results and discussions Conductivity of superplasticizers
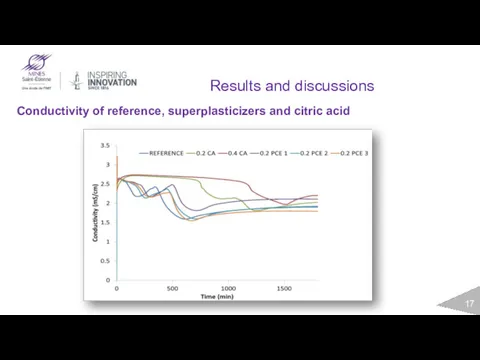
- 17. Results and discussions Conductivity of reference, superplasticizers and citric acid
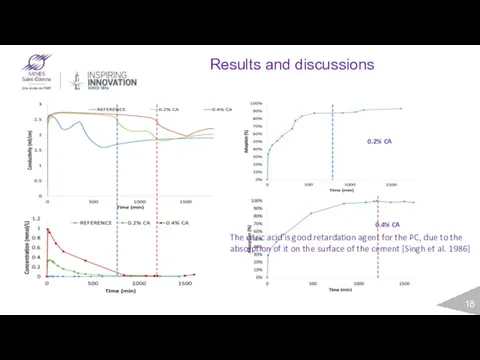
- 18. Results and discussions 0.2% CA 0.4% CA The citric acid is good retardation agent for the
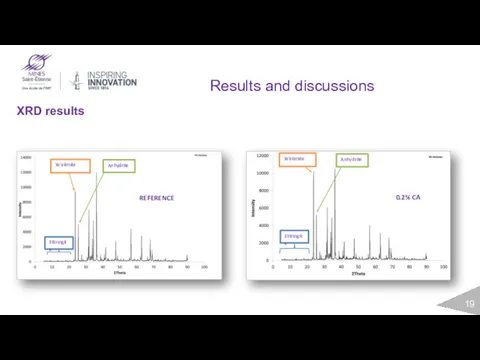
- 19. Results and discussions XRD results Ettringite Ye’elimite Anhydrite REFERENCE Ettringite Ye’elimite Anhydrite 0.2% CA
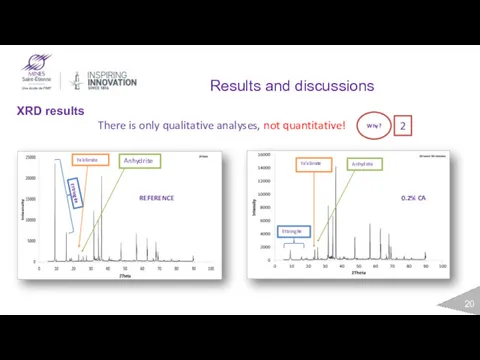
- 20. Results and discussions XRD results Ettringite Ye’elimite Anhydrite REFERENCE Ye’elimite Anhydrite Ettringite 0.2% CA There is
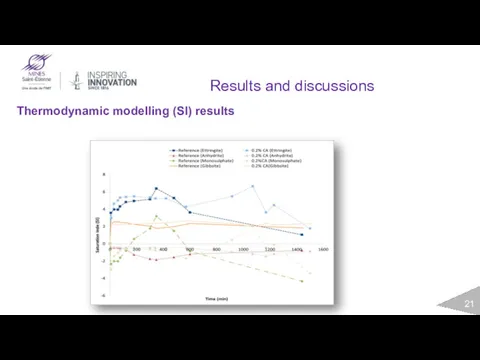
- 21. Results and discussions Thermodynamic modelling (SI) results
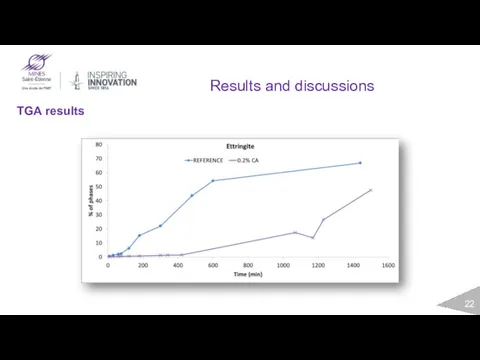
- 22. Results and discussions TGA results
- 23. Conclusion Conductivity analyses Reference 0.1-0.3% PCE 1 0.1-0.3% PCE 2 0.1-0.3% PCE 3 0.2% and 0.4%
- 24. Conclusion 2 1 Sulfur Ye’elimite
- 26. Скачать презентацию









 портфолио профессиональной деятельности
портфолио профессиональной деятельности fcd75ffc67cf3c64
fcd75ffc67cf3c64 Презентация 14. Пейзаж второй половины XIX в. Часть 2_ Васильев, Куинджи
Презентация 14. Пейзаж второй половины XIX в. Часть 2_ Васильев, Куинджи Игрушка-дергунчик своими руками
Игрушка-дергунчик своими руками Развитие сенсорных способностей посредством дидактических игр
Развитие сенсорных способностей посредством дидактических игр Сертификация. Оценка соответствия
Сертификация. Оценка соответствия Краеведение, как условие духовно-нравственного воспитания школьников
Краеведение, как условие духовно-нравственного воспитания школьников Презентация к внеклассному занятию Домашние птицы
Презентация к внеклассному занятию Домашние птицы WI-FI для бизнеса
WI-FI для бизнеса Многоплодная беременность. Профессиональная роль акушерки
Многоплодная беременность. Профессиональная роль акушерки Игра для детей 3-4 лет. Правила дорожного движения
Игра для детей 3-4 лет. Правила дорожного движения Организация, биология и происхождение наземных Позвоночных. Анамнии и Амниоты. Амфибии, особенности строения
Организация, биология и происхождение наземных Позвоночных. Анамнии и Амниоты. Амфибии, особенности строения Физиолого-генетический подход к вопросам спортивного отбора
Физиолого-генетический подход к вопросам спортивного отбора Город будущего
Город будущего Занятие Введение в программу
Занятие Введение в программу Признаки параллельности прямых. Задачи
Признаки параллельности прямых. Задачи Значение, задачи и методический инструментарий экономического анализа
Значение, задачи и методический инструментарий экономического анализа Еңбек гигиенасы, коммуналдық гигиена және ТҚН Еңбек гигиенасындағы нанотехнологиялар
Еңбек гигиенасы, коммуналдық гигиена және ТҚН Еңбек гигиенасындағы нанотехнологиялар Презинтация
Презинтация Заповедники и парки России
Заповедники и парки России Классификация звуков речи. Система фонем
Классификация звуков речи. Система фонем Повторение теории и решение тригонометрических уравнений в рамках подготовки к ЕГЭ
Повторение теории и решение тригонометрических уравнений в рамках подготовки к ЕГЭ Простейшие грузоподъемные устройства и механизмы. Лекция 2
Простейшие грузоподъемные устройства и механизмы. Лекция 2 Zvuk. Infrazvuk
Zvuk. Infrazvuk Толерантность
Толерантность Материал на конкурс презентаций трудовых династий Имя свое крепи делами своими Тематика конкурсного материала: Старейшая трудовая династия
Материал на конкурс презентаций трудовых династий Имя свое крепи делами своими Тематика конкурсного материала: Старейшая трудовая династия Школа моей мечты
Школа моей мечты Овощи
Овощи