Содержание
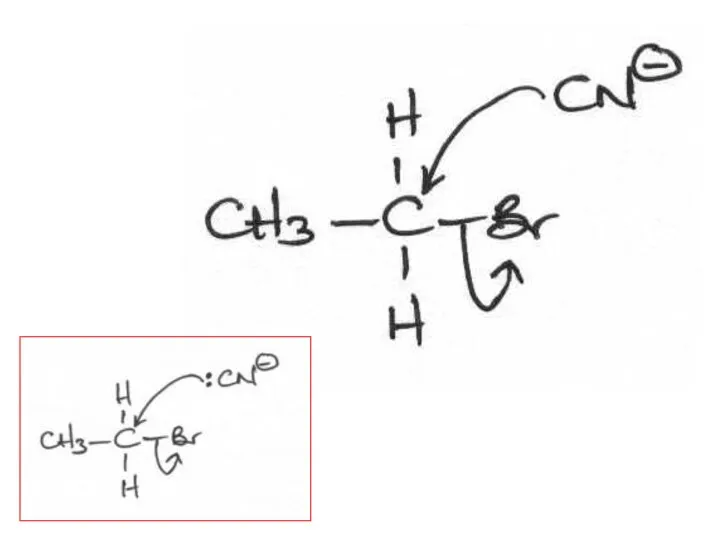
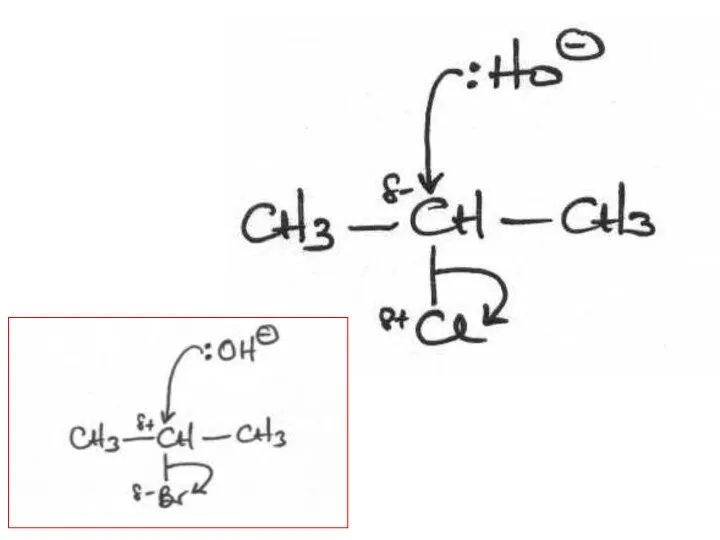
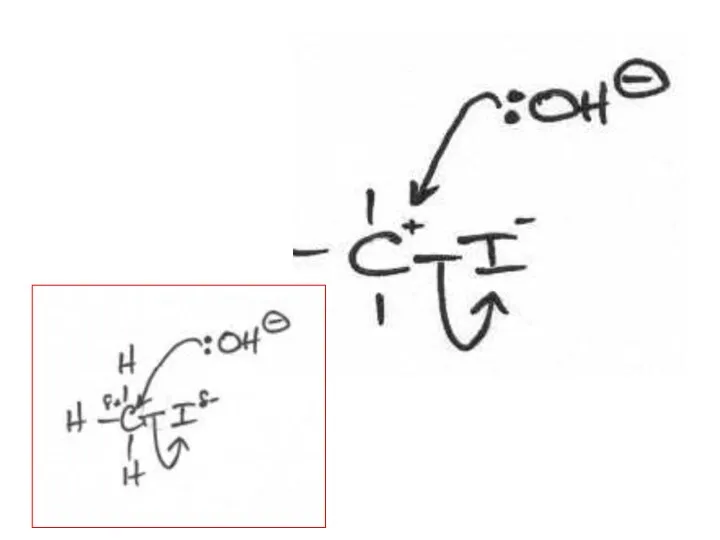
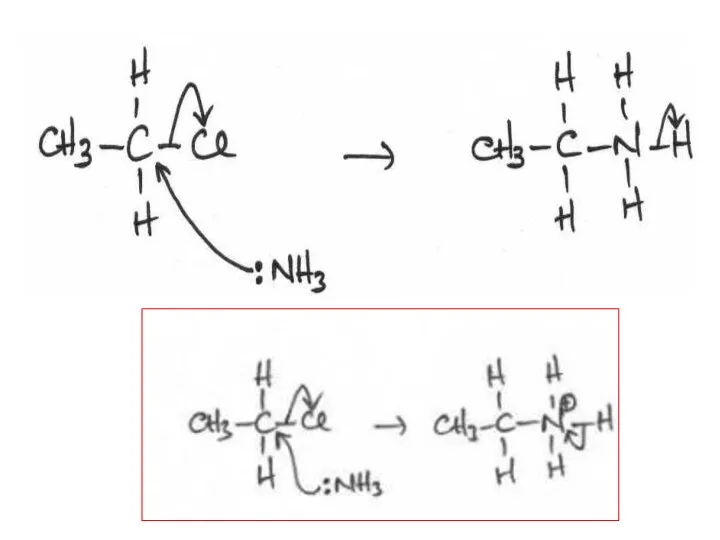
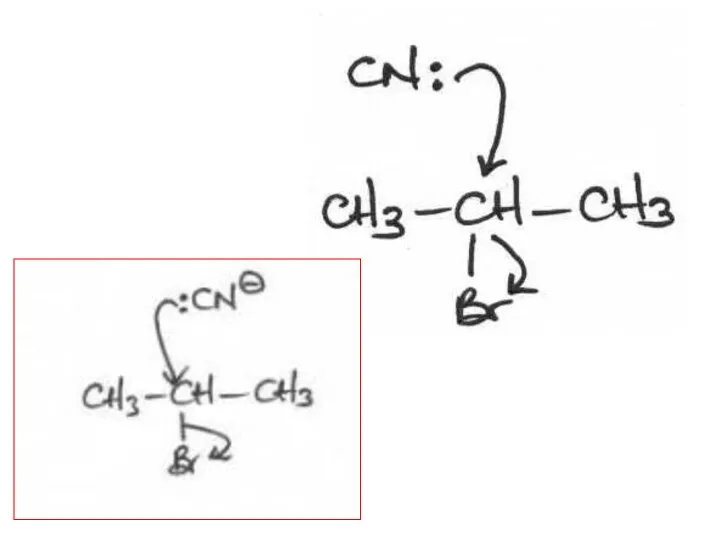
- 2. The following mechanisms have some mistakes. Find out the mistake(s) and have it (them) corrected. Review
- 8. Learning Objectives Understand elimination and its mechanism Understand the competition between substitution and elimination Understand the
- 9. Success Criteria Predict the product(s) of elimination reactions in halogenoalkanes. Outline the mechanism for elimination reactions
- 10. Keywords Elimination reaction Nucleophilic substitution Nucleophile Base ( H+ proton acceptor) Zaitsev’s rule Reflux
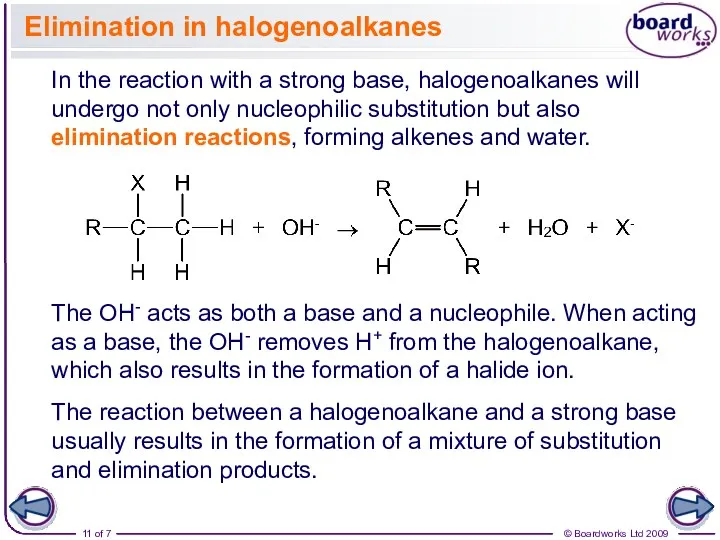
- 11. Elimination in halogenoalkanes In the reaction with a strong base, halogenoalkanes will undergo not only nucleophilic
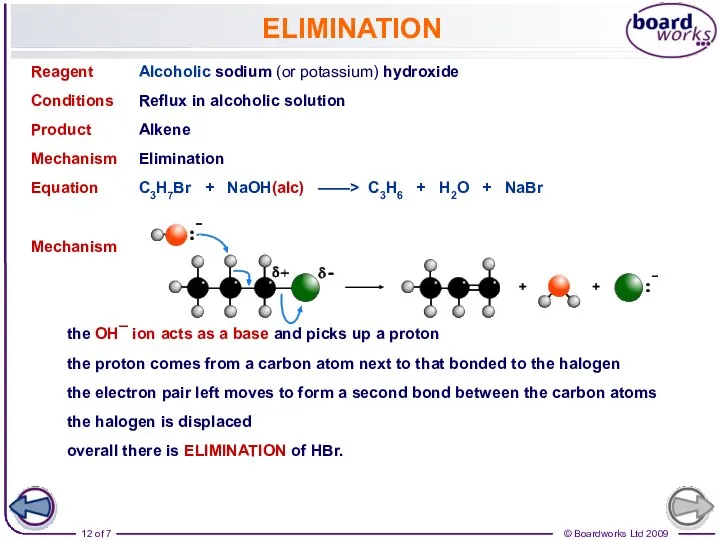
- 12. ELIMINATION Reagent Alcoholic sodium (or potassium) hydroxide Conditions Reflux in alcoholic solution Product Alkene Mechanism Elimination
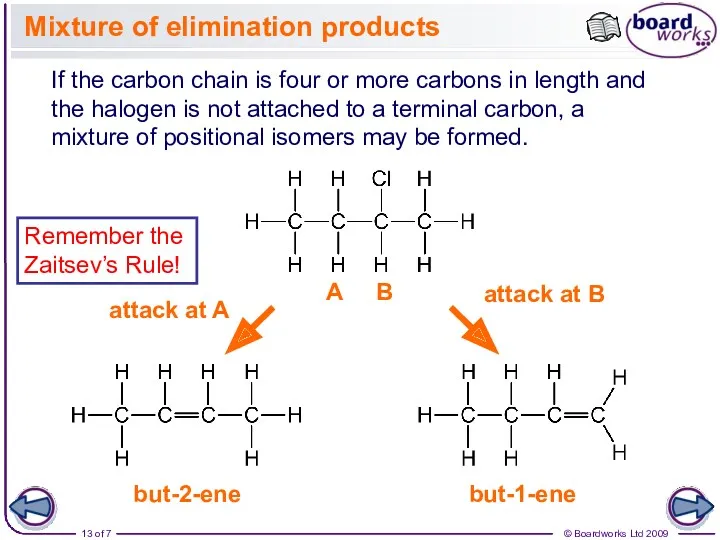
- 13. Mixture of elimination products If the carbon chain is four or more carbons in length and
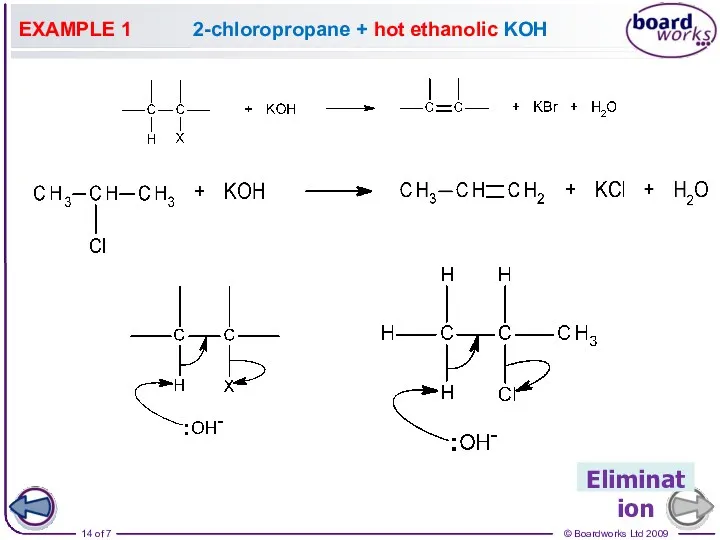
- 14. EXAMPLE 1 2-chloropropane + hot ethanolic KOH Elimination
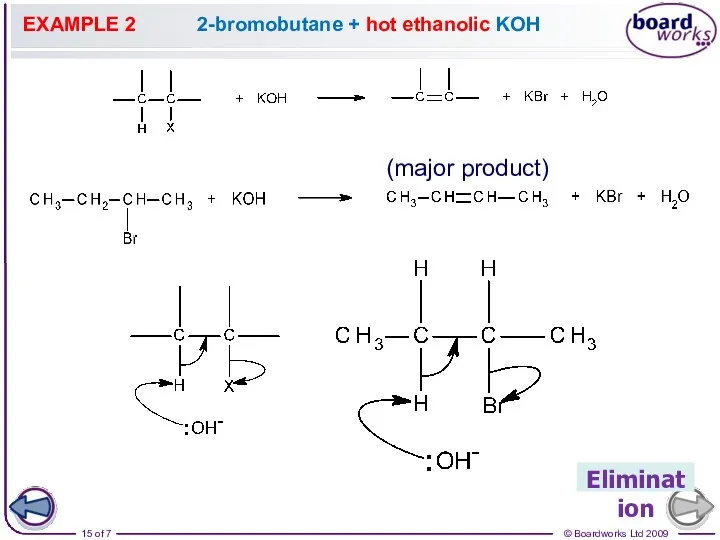
- 15. EXAMPLE 2 2-bromobutane + hot ethanolic KOH Elimination (major product)
- 16. Conditions are important The conditions for the reaction that favour substitution or elimination are different. Base
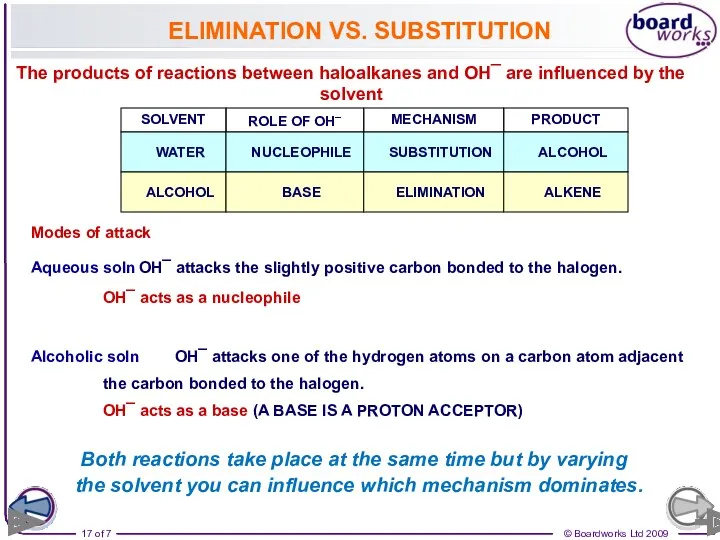
- 17. ELIMINATION VS. SUBSTITUTION The products of reactions between haloalkanes and OH¯ are influenced by the solvent
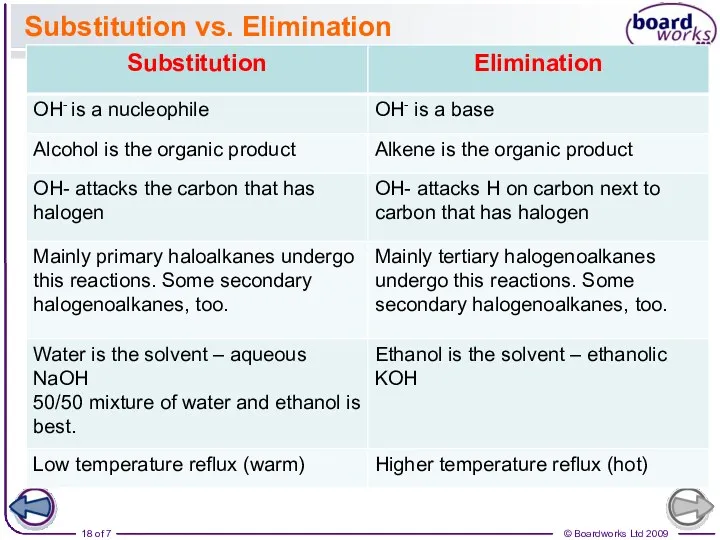
- 18. Substitution vs. Elimination
- 19. Halogenoalkanes as intermediates As well as being useful in their own right, halogenoalkanes are important intermediates
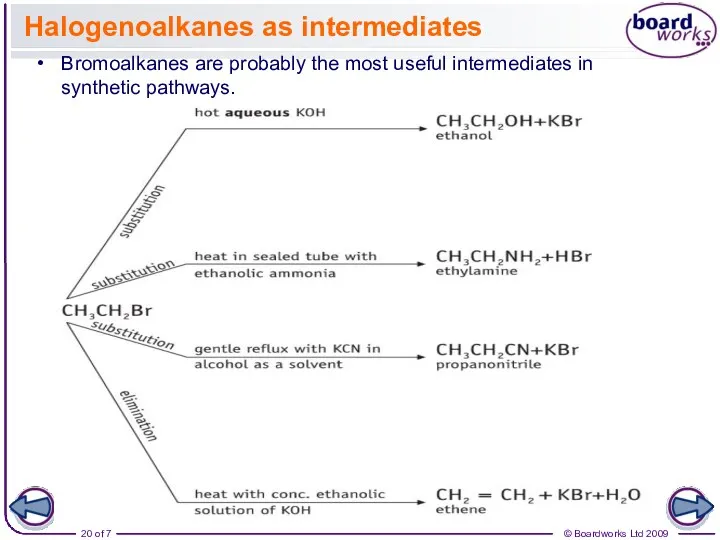
- 20. Halogenoalkanes as intermediates Bromoalkanes are probably the most useful intermediates in synthetic pathways.
- 21. Understand elimination and its mechanism Understand the competition between substitution and elimination Understand the importance of
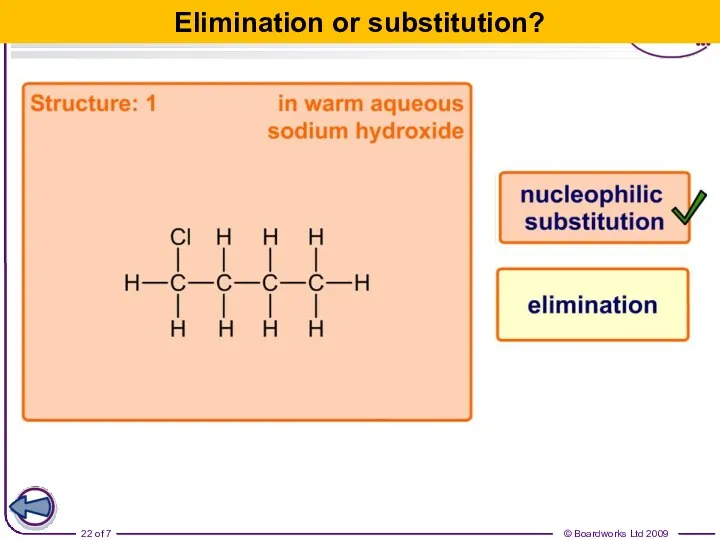
- 22. Elimination or substitution?
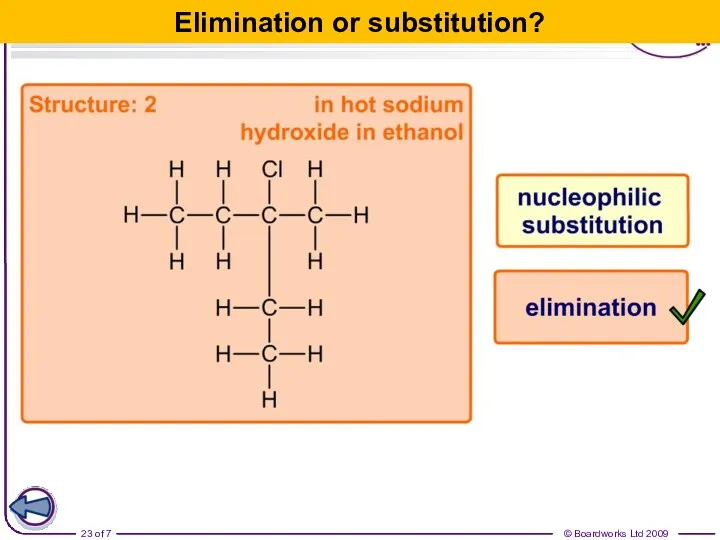
- 23. Elimination or substitution?
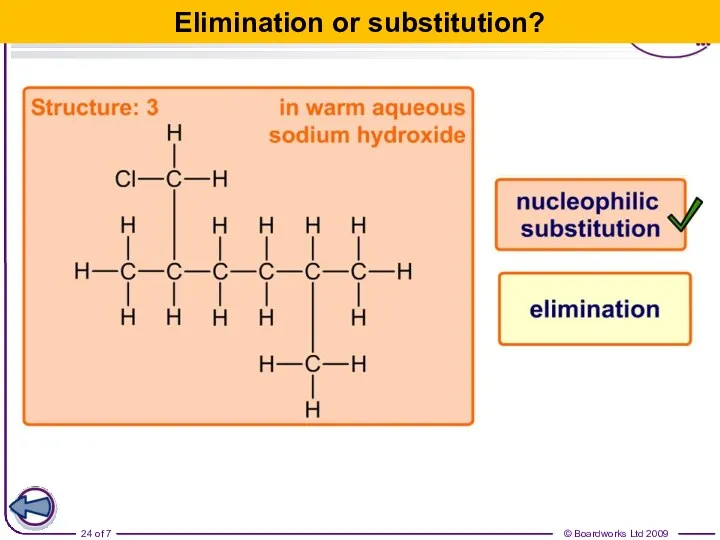
- 24. Elimination or substitution?
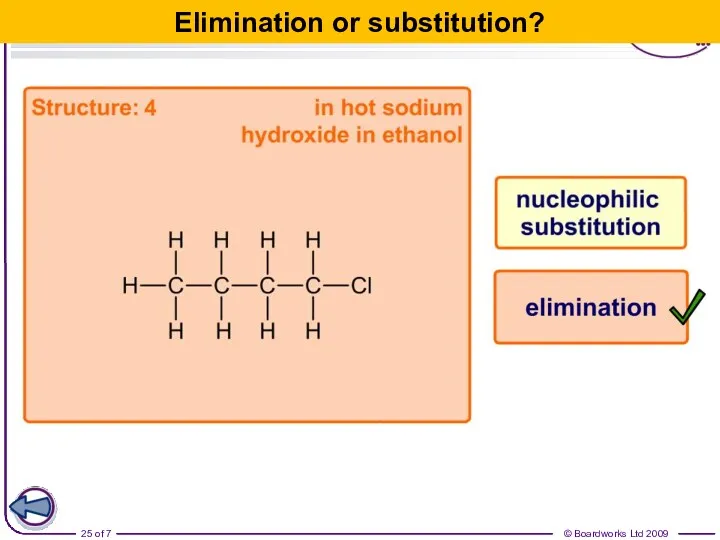
- 25. Elimination or substitution?
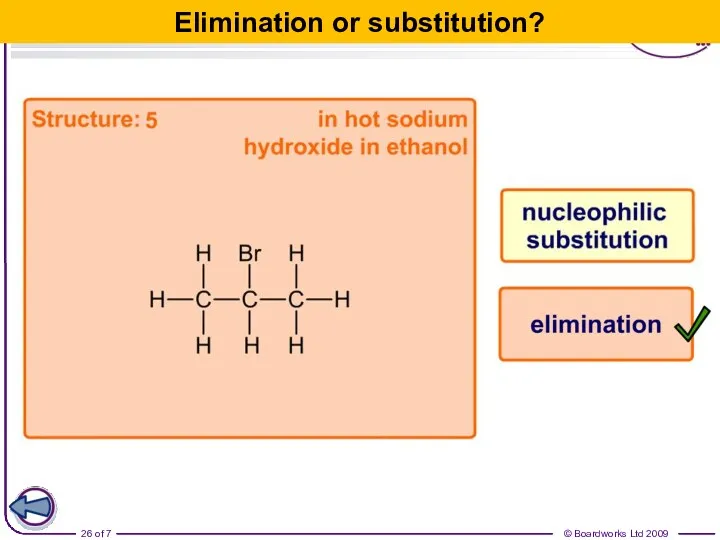
- 26. Elimination or substitution?
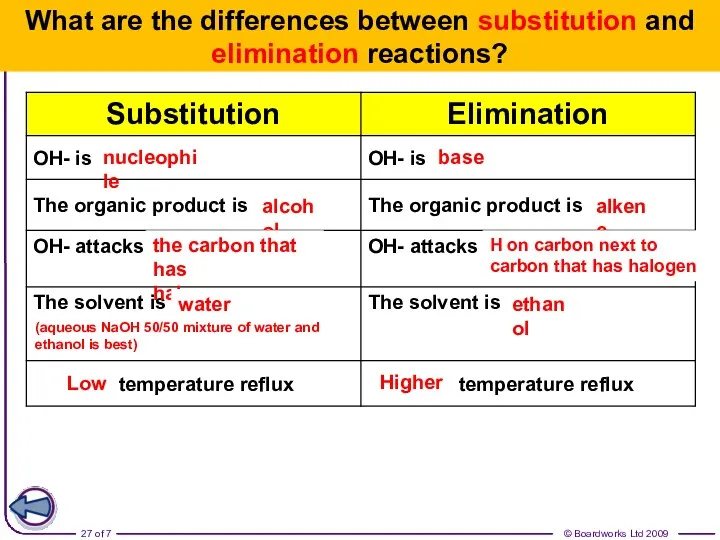
- 27. What are the differences between substitution and elimination reactions? nucleophile base alcohol alkene the carbon that
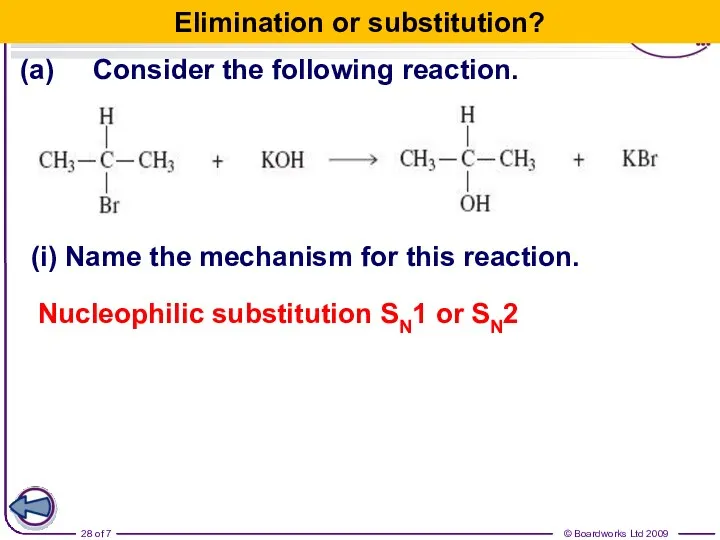
- 28. (a) Consider the following reaction. (i) Name the mechanism for this reaction. Nucleophilic substitution SN1 or
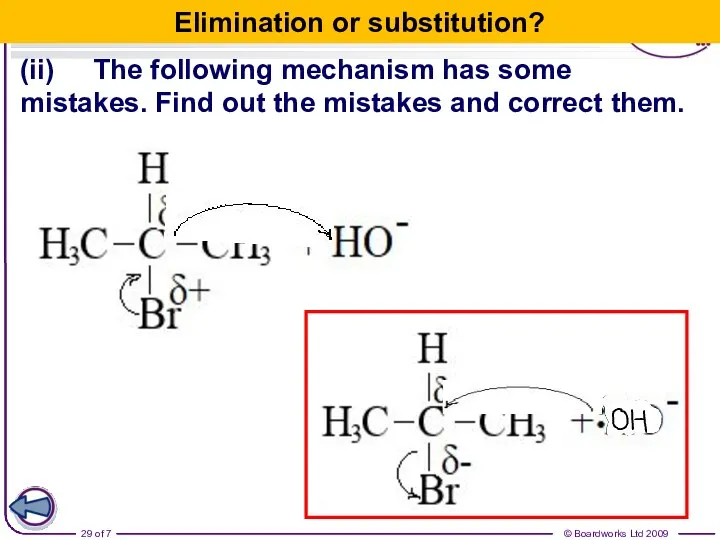
- 29. (ii) The following mechanism has some mistakes. Find out the mistakes and correct them. Elimination or
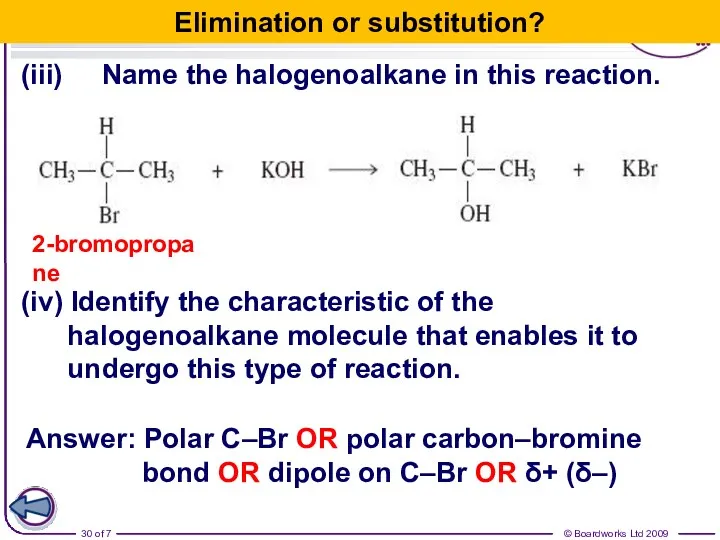
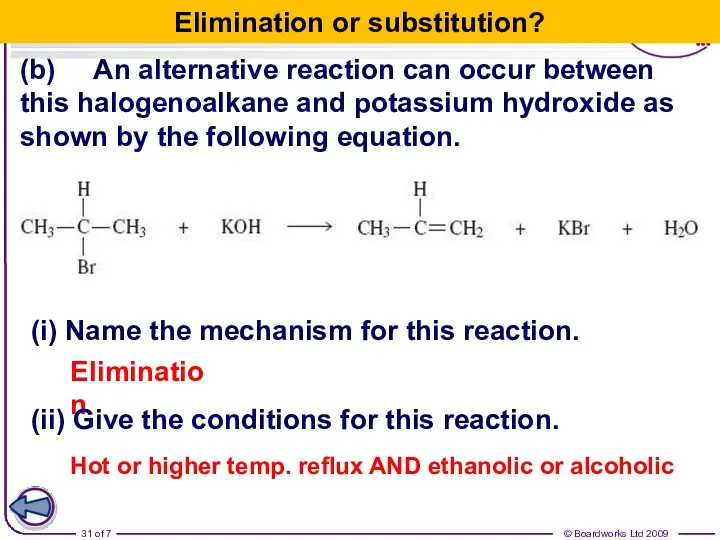
- 30. (iii) Name the halogenoalkane in this reaction. 2-bromopropane (iv) Identify the characteristic of the halogenoalkane molecule
- 31. (b) An alternative reaction can occur between this halogenoalkane and potassium hydroxide as shown by the
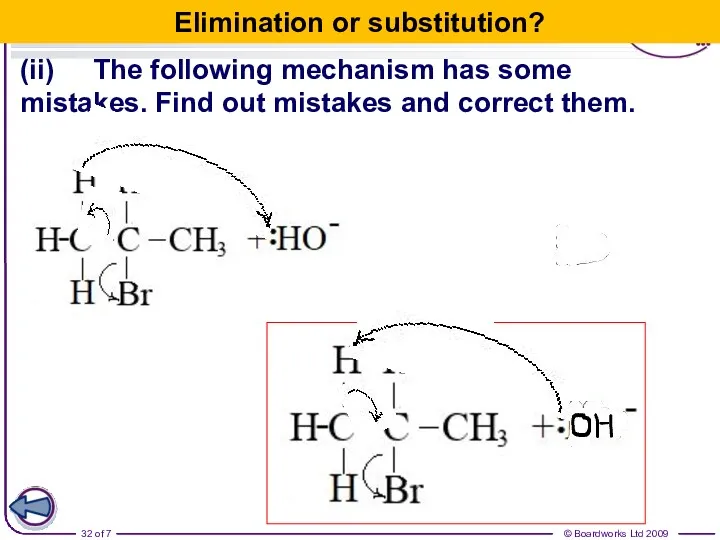
- 32. (ii) The following mechanism has some mistakes. Find out mistakes and correct them. Elimination or substitution?
- 34. Скачать презентацию





 Влияние загрязнения окружающей среды на здоровье
Влияние загрязнения окружающей среды на здоровье Презентация к статье Я-сетевой преподаватель
Презентация к статье Я-сетевой преподаватель Горные породы и минералы.
Горные породы и минералы. Месторождения хрома
Месторождения хрома Асинхронные машины. Лекция 13
Асинхронные машины. Лекция 13 Л 3 пределы последовательностей с ФАКТОРИАЛом
Л 3 пределы последовательностей с ФАКТОРИАЛом 20230721_slozhnopodchinyonnye_predlozheniya
20230721_slozhnopodchinyonnye_predlozheniya Многопрофильная клиника ООО Медицинский центр жизнь
Многопрофильная клиника ООО Медицинский центр жизнь Раскрой швейного изделия
Раскрой швейного изделия Городской конкурс проектов Мы любим свой город
Городской конкурс проектов Мы любим свой город Арабо-мусульманская культура
Арабо-мусульманская культура Технология изготовления бочек
Технология изготовления бочек Вегетарианство: польза или вред для организма. 9 класс
Вегетарианство: польза или вред для организма. 9 класс Закаливание организма
Закаливание организма Plurals
Plurals Классный час Мир моих увлечений 2-3 класс
Классный час Мир моих увлечений 2-3 класс Безопасность в учреждениях образования
Безопасность в учреждениях образования Потенциальная помехоустойчивость. Лекции №3
Потенциальная помехоустойчивость. Лекции №3 1 сентября - День знаний 4 класс
1 сентября - День знаний 4 класс Диплом_Петров
Диплом_Петров Форми і елементи управління форм. Теги для роботи з формами
Форми і елементи управління форм. Теги для роботи з формами Простые вещества, металлы
Простые вещества, металлы Проектирование реакционного узла для жидкофазных реакторов. Лекция 5
Проектирование реакционного узла для жидкофазных реакторов. Лекция 5 Развитие государственно-частного партнерства в дорожном секторе транспортной отрасли России
Развитие государственно-частного партнерства в дорожном секторе транспортной отрасли России Троица. Закон Божий для семьи и школы
Троица. Закон Божий для семьи и школы Особенности делового общения с иностранными партнерами
Особенности делового общения с иностранными партнерами ПРЕЗЕНТАЦИЯ ДЛЯ ИНТЕРАКТИВНОЙ ДОСКИ. Терриконы 9 кл.
ПРЕЗЕНТАЦИЯ ДЛЯ ИНТЕРАКТИВНОЙ ДОСКИ. Терриконы 9 кл. Смысл названия пьеса А.Н.Островского “Гроза”
Смысл названия пьеса А.Н.Островского “Гроза”