Содержание
- 2. Lithium (Li/MnO2 in KOH) CATEGORY: Primary (Throwaway) Lithium Family CONSTRUCTION: Very similar to alkaline cell in
- 3. Mercury (Zn/HgO in KOH) CATEGORY: Primary (Throwaway) Zinc Family CONSTRUCTION: A mercury battery. A zinc-mercury amalgam
- 4. Silver oxide (Zn/Ag2O in KOH) CATEGORY: Primary (Throwaway) Zinc Family CONSTRUCTION: A silver oxide button battery,
- 5. Zinc-Air (Zn/O2 in KOH) CATEGORY: Primary (Throwaway) Zinc Family CONSTRUCTION: Constructions very similar to mercury or
- 7. Скачать презентацию
Слайд 2
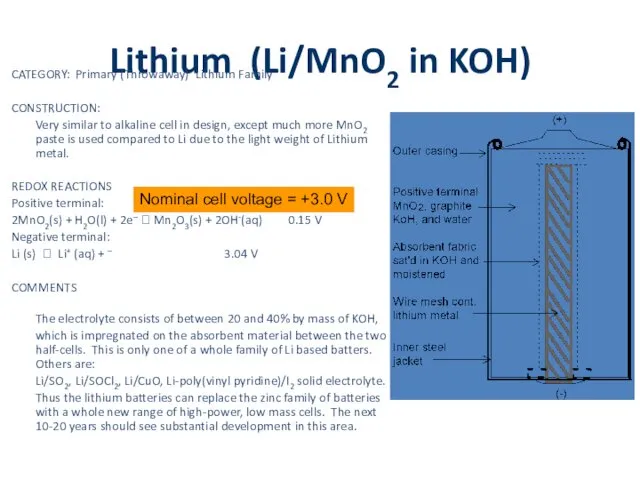
Lithium (Li/MnO2 in KOH)
CATEGORY: Primary (Throwaway) Lithium Family
CONSTRUCTION:
Very similar to alkaline cell
Lithium (Li/MnO2 in KOH)
CATEGORY: Primary (Throwaway) Lithium Family
CONSTRUCTION:
Very similar to alkaline cell
in design, except much more MnO2 paste is used compared to Li due to the light weight of Lithium metal.
REDOX REACTIONS
Positive terminal:
2MnO2(s) + H2O(l) + 2e– ? Mn2O3(s) + 2OH-(aq) 0.15 V
Negative terminal:
Li (s) ? Li+ (aq) + – 3.04 V
COMMENTS
The electrolyte consists of between 20 and 40% by mass of KOH, which is impregnated on the absorbent material between the two half-cells. This is only one of a whole family of Li based batters. Others are:
Li/SO2, Li/SOCl2, Li/CuO, Li-poly(vinyl pyridine)/I2 solid electrolyte.
Thus the lithium batteries can replace the zinc family of batteries with a whole new range of high-power, low mass cells. The next 10-20 years should see substantial development in this area.
REDOX REACTIONS
Positive terminal:
2MnO2(s) + H2O(l) + 2e– ? Mn2O3(s) + 2OH-(aq) 0.15 V
Negative terminal:
Li (s) ? Li+ (aq) + – 3.04 V
COMMENTS
The electrolyte consists of between 20 and 40% by mass of KOH, which is impregnated on the absorbent material between the two half-cells. This is only one of a whole family of Li based batters. Others are:
Li/SO2, Li/SOCl2, Li/CuO, Li-poly(vinyl pyridine)/I2 solid electrolyte.
Thus the lithium batteries can replace the zinc family of batteries with a whole new range of high-power, low mass cells. The next 10-20 years should see substantial development in this area.
Nominal cell voltage = +3.0 V
Слайд 3
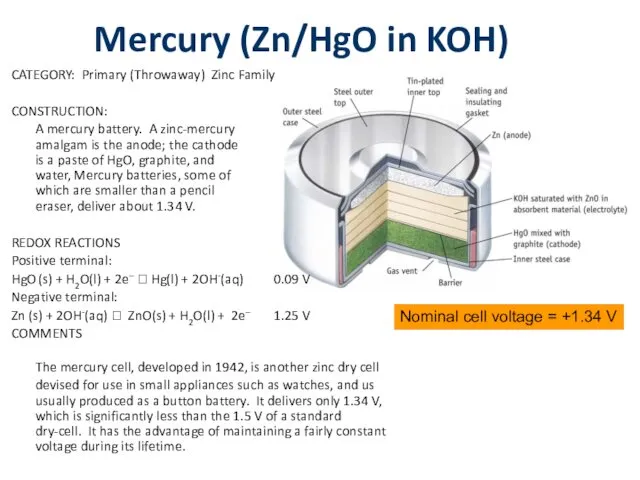
Mercury (Zn/HgO in KOH)
CATEGORY: Primary (Throwaway) Zinc Family
CONSTRUCTION:
A mercury battery. A
Mercury (Zn/HgO in KOH)
CATEGORY: Primary (Throwaway) Zinc Family
CONSTRUCTION:
A mercury battery. A
zinc-mercury
amalgam is the anode; the cathode
is a paste of HgO, graphite, and
water, Mercury batteries, some of
which are smaller than a pencil
eraser, deliver about 1.34 V.
REDOX REACTIONS
Positive terminal:
HgO (s) + H2O(l) + 2e– ? Hg(l) + 2OH-(aq) 0.09 V
Negative terminal:
Zn (s) + 2OH-(aq) ? ZnO(s) + H2O(l) + 2e– 1.25 V
COMMENTS
The mercury cell, developed in 1942, is another zinc dry cell devised for use in small appliances such as watches, and us usually produced as a button battery. It delivers only 1.34 V, which is significantly less than the 1.5 V of a standard dry-cell. It has the advantage of maintaining a fairly constant voltage during its lifetime.
REDOX REACTIONS
Positive terminal:
HgO (s) + H2O(l) + 2e– ? Hg(l) + 2OH-(aq) 0.09 V
Negative terminal:
Zn (s) + 2OH-(aq) ? ZnO(s) + H2O(l) + 2e– 1.25 V
COMMENTS
The mercury cell, developed in 1942, is another zinc dry cell devised for use in small appliances such as watches, and us usually produced as a button battery. It delivers only 1.34 V, which is significantly less than the 1.5 V of a standard dry-cell. It has the advantage of maintaining a fairly constant voltage during its lifetime.
Nominal cell voltage = +1.34 V
Слайд 4
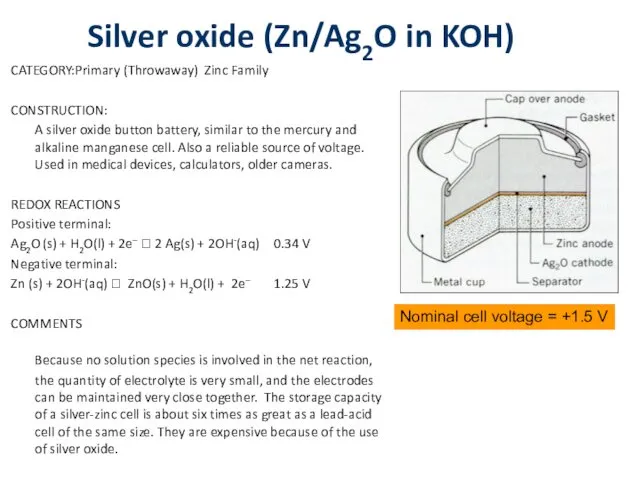
Silver oxide (Zn/Ag2O in KOH)
CATEGORY: Primary (Throwaway) Zinc Family
CONSTRUCTION:
A silver oxide button
Silver oxide (Zn/Ag2O in KOH)
CATEGORY: Primary (Throwaway) Zinc Family
CONSTRUCTION:
A silver oxide button
battery, similar to the mercury and alkaline manganese cell. Also a reliable source of voltage. Used in medical devices, calculators, older cameras.
REDOX REACTIONS
Positive terminal:
Ag2O (s) + H2O(l) + 2e– ? 2 Ag(s) + 2OH-(aq) 0.34 V
Negative terminal:
Zn (s) + 2OH-(aq) ? ZnO(s) + H2O(l) + 2e– 1.25 V
COMMENTS
Because no solution species is involved in the net reaction, the quantity of electrolyte is very small, and the electrodes can be maintained very close together. The storage capacity of a silver-zinc cell is about six times as great as a lead-acid cell of the same size. They are expensive because of the use of silver oxide.
REDOX REACTIONS
Positive terminal:
Ag2O (s) + H2O(l) + 2e– ? 2 Ag(s) + 2OH-(aq) 0.34 V
Negative terminal:
Zn (s) + 2OH-(aq) ? ZnO(s) + H2O(l) + 2e– 1.25 V
COMMENTS
Because no solution species is involved in the net reaction, the quantity of electrolyte is very small, and the electrodes can be maintained very close together. The storage capacity of a silver-zinc cell is about six times as great as a lead-acid cell of the same size. They are expensive because of the use of silver oxide.
Nominal cell voltage = +1.5 V
Слайд 5
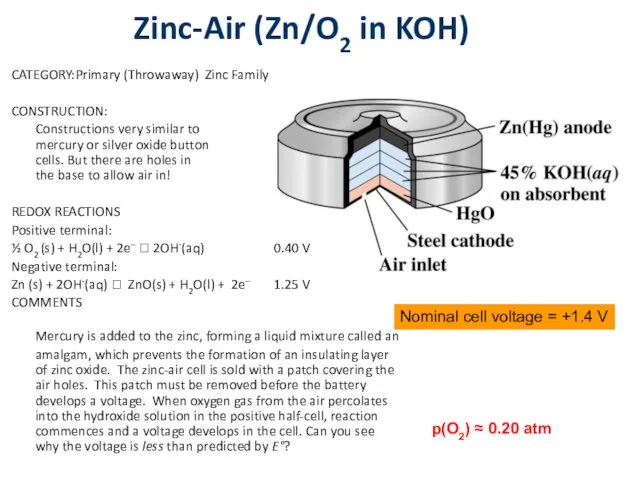
Zinc-Air (Zn/O2 in KOH)
CATEGORY: Primary (Throwaway) Zinc Family
CONSTRUCTION:
Constructions very similar to
mercury or
Zinc-Air (Zn/O2 in KOH)
CATEGORY: Primary (Throwaway) Zinc Family
CONSTRUCTION:
Constructions very similar to
mercury or
silver oxide button
cells. But there are holes in
the base to allow air in!
REDOX REACTIONS
Positive terminal:
½ O2 (s) + H2O(l) + 2e– ? 2OH-(aq) 0.40 V
Negative terminal:
Zn (s) + 2OH-(aq) ? ZnO(s) + H2O(l) + 2e– 1.25 V
COMMENTS
Mercury is added to the zinc, forming a liquid mixture called an amalgam, which prevents the formation of an insulating layer of zinc oxide. The zinc-air cell is sold with a patch covering the air holes. This patch must be removed before the battery develops a voltage. When oxygen gas from the air percolates into the hydroxide solution in the positive half-cell, reaction commences and a voltage develops in the cell. Can you see why the voltage is less than predicted by E°?
REDOX REACTIONS
Positive terminal:
½ O2 (s) + H2O(l) + 2e– ? 2OH-(aq) 0.40 V
Negative terminal:
Zn (s) + 2OH-(aq) ? ZnO(s) + H2O(l) + 2e– 1.25 V
COMMENTS
Mercury is added to the zinc, forming a liquid mixture called an amalgam, which prevents the formation of an insulating layer of zinc oxide. The zinc-air cell is sold with a patch covering the air holes. This patch must be removed before the battery develops a voltage. When oxygen gas from the air percolates into the hydroxide solution in the positive half-cell, reaction commences and a voltage develops in the cell. Can you see why the voltage is less than predicted by E°?
Nominal cell voltage = +1.4 V
p(O2) ≈ 0.20 atm
 Атомная энергетика. Принцип работы АЭС
Атомная энергетика. Принцип работы АЭС Роль испарения и конденсации в природе, в жизни человека и животных
Роль испарения и конденсации в природе, в жизни человека и животных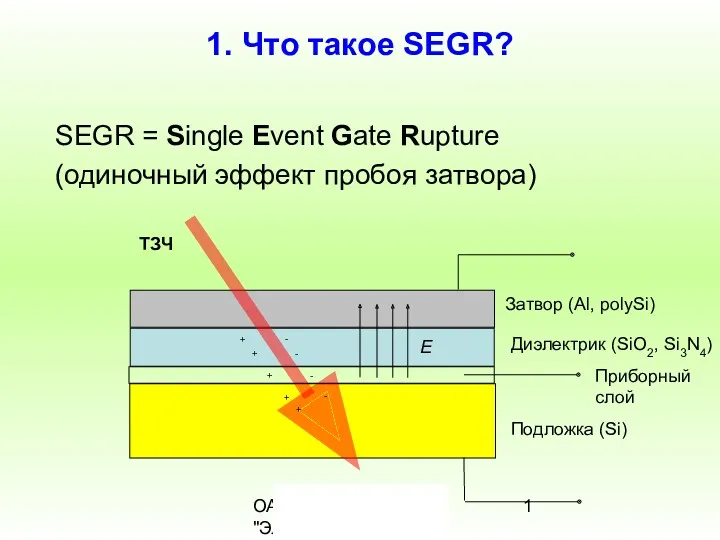 Что такое SEGR (одиночный эффект пробоя затвора)
Что такое SEGR (одиночный эффект пробоя затвора) 1D PIC code for TwoStream Plasma Instability
1D PIC code for TwoStream Plasma Instability Испытания БЗО торпеды на безопасность при воздействии теплового поля пожара
Испытания БЗО торпеды на безопасность при воздействии теплового поля пожара электрическое поле. напряжонность
электрическое поле. напряжонность Electricity. Learning Outcome
Electricity. Learning Outcome Закон Архимеда
Закон Архимеда Механік - моя спеціальність
Механік - моя спеціальність Сила упругости. Закон Гука
Сила упругости. Закон Гука Силы в природе. Обобщающий урок. 7 класс
Силы в природе. Обобщающий урок. 7 класс Электромагниты. Применение электромагнитов
Электромагниты. Применение электромагнитов Работа силы. Мощность
Работа силы. Мощность Общие сведения об интерференции света
Общие сведения об интерференции света Химические реакторы. Гетерогенно-каталитические химические процессы. Лекция №12
Химические реакторы. Гетерогенно-каталитические химические процессы. Лекция №12 Агрегатные состояния вещества
Агрегатные состояния вещества Магнитные свойства вещества
Магнитные свойства вещества Первый закон термодинамики. Тепловые двигатели и КПД тепловых двигателей
Первый закон термодинамики. Тепловые двигатели и КПД тепловых двигателей Разработка производственной программы для станции технического обслуживания с разработкой технологии тюнинга
Разработка производственной программы для станции технического обслуживания с разработкой технологии тюнинга Управление нефтегазовыми технологическими процессами
Управление нефтегазовыми технологическими процессами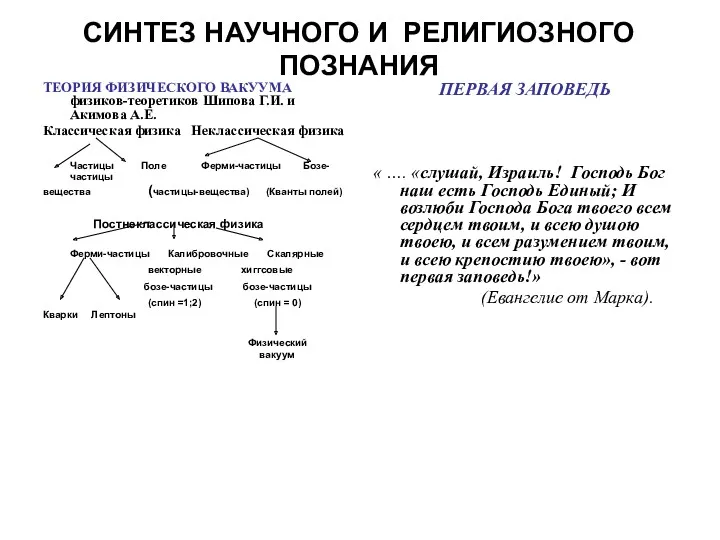 Синтез научного и религиозного познания. Теория физического вакуума
Синтез научного и религиозного познания. Теория физического вакуума Cummins Engine Company LTD
Cummins Engine Company LTD Кинематика точки (Лекция 1, кафедра теоретической механики )
Кинематика точки (Лекция 1, кафедра теоретической механики ) Постоянный электрический ток
Постоянный электрический ток Основы теории напряженного состояния. Понятия главных площадок и главных напряжений. Лекция 9
Основы теории напряженного состояния. Понятия главных площадок и главных напряжений. Лекция 9 Ремонт автомобилей. Ремонт кузовов и кабин. (Тема 4.11)
Ремонт автомобилей. Ремонт кузовов и кабин. (Тема 4.11) Обобщение и систематизация основных знаний по теме Электрические явления. Законы постоянного тока
Обобщение и систематизация основных знаний по теме Электрические явления. Законы постоянного тока Презентация к уроку на тему: Великий ученый древнего мира – Архимед и его закон
Презентация к уроку на тему: Великий ученый древнего мира – Архимед и его закон