Содержание
- 2. Properties of solutions that depend on the number of molecules present and not on the kind
- 3. Raoult’s Law: Fractional lowering of the saturated vapor pressure of a solvent above a solution is
- 4. The freezing point of a nonvolatile substance solution is always lower than the freezing point of
- 5. Spontaneous process of solute concentration leveling in the whole volume of the solution, due to the
- 6. One-side diffusion of solvent molecules through a semipermeable membrane to a more concentrated solution is called
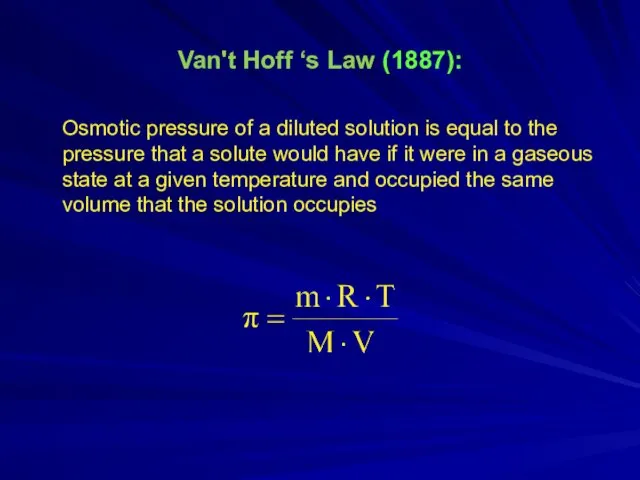
- 7. Van't Hoff ‘s Law (1887): Osmotic pressure of a diluted solution is equal to the pressure
- 8. Turgor is a state of tension of the cellular cover caused by osmotic pressure of the
- 9. Solutions with an identical osmotic pressure are called isotonic. Solutions with a higher osmotic pressure than
- 11. Скачать презентацию







 Разгонные блоки космических аппаратов
Разгонные блоки космических аппаратов Делительные головки
Делительные головки Chemical energy storage
Chemical energy storage Атом. 9 класс
Атом. 9 класс Основы термодинамики. Решение задач
Основы термодинамики. Решение задач Подсистемы системы Корабль. Подсистема Маневрирование
Подсистемы системы Корабль. Подсистема Маневрирование Гидравлический расчет кольцевой водопроводной сети
Гидравлический расчет кольцевой водопроводной сети Давление. Закон Архимеда. Контрольная работа
Давление. Закон Архимеда. Контрольная работа практическая работа по теме Линзы в 11 классе
практическая работа по теме Линзы в 11 классе Урок: Параллельное соединение проводников
Урок: Параллельное соединение проводников Свободное падение
Свободное падение 8. Плоскопараллельное движение твердого тела (плоское)
8. Плоскопараллельное движение твердого тела (плоское) Проект Экзофиз
Проект Экзофиз Микроскопия, виды и возможности современных микроскопов
Микроскопия, виды и возможности современных микроскопов Динамика вращательного движения
Динамика вращательного движения Элементы машиноведения. Составные части машин
Элементы машиноведения. Составные части машин Всероссийская олимпиада школьников по физике. Разбор заданий муниципального этапа. 11 класс
Всероссийская олимпиада школьников по физике. Разбор заданий муниципального этапа. 11 класс История возниктовения электрического освещения
История возниктовения электрического освещения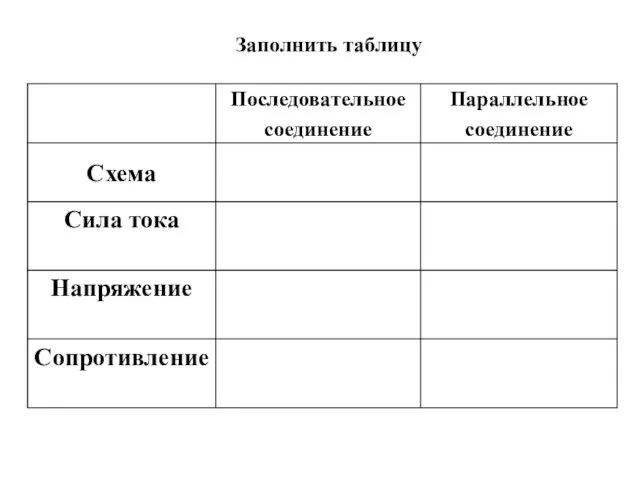 Последовательное соединение проводников
Последовательное соединение проводников Анализ и перспективы развития элегазового и вакуумного оборудования подстанций энергосистемы
Анализ и перспективы развития элегазового и вакуумного оборудования подстанций энергосистемы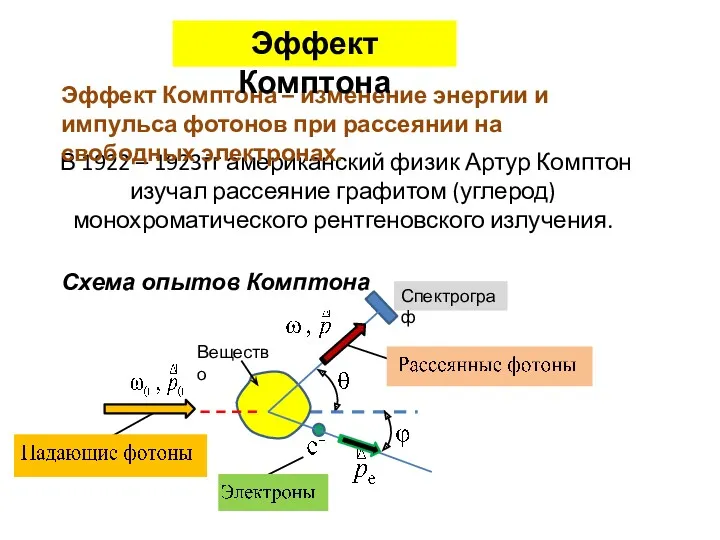 Эффект Комптона
Эффект Комптона Оливин. Физические и химические свойства
Оливин. Физические и химические свойства “Движение тел. Материальная точка”
“Движение тел. Материальная точка” Атомный фактор рассеяния. Рассеяние рентгеновских лучей на электронах в атомах
Атомный фактор рассеяния. Рассеяние рентгеновских лучей на электронах в атомах Леонардо Да Винчи и его технические изобретения
Леонардо Да Винчи и его технические изобретения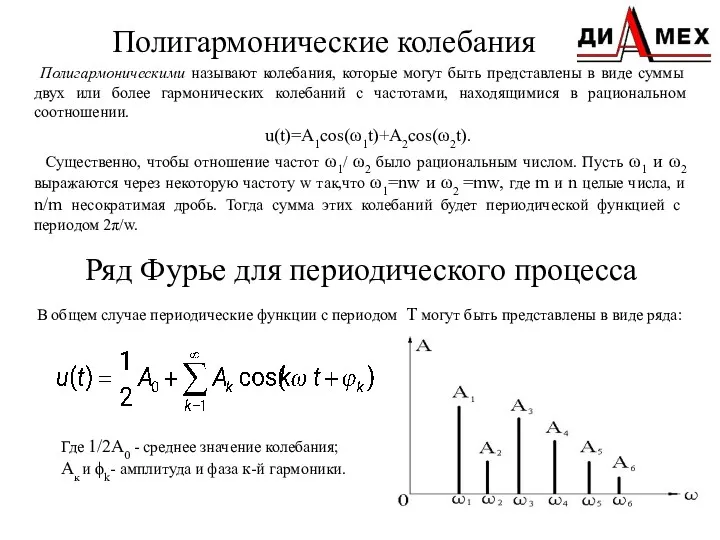 Полигармонические колебания
Полигармонические колебания Газ заңдары
Газ заңдары Закон Ома для полной цепи
Закон Ома для полной цепи