Содержание
- 2. The course is based on: Basic operation of cryocoolers and related thermal machines A.T.A.M. de Waele
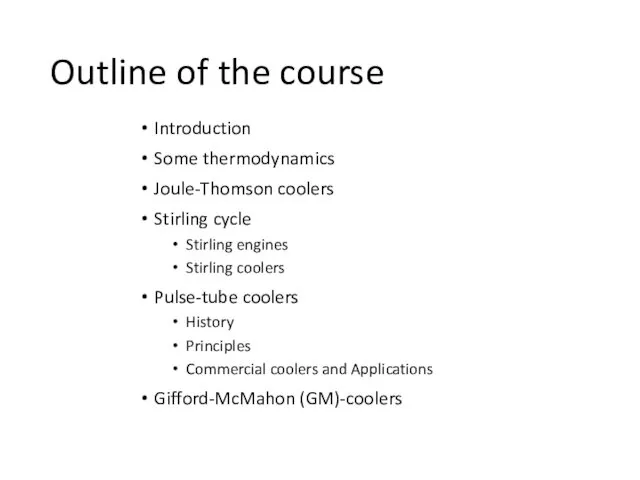
- 3. Outline of the course Introduction Some thermodynamics Joule-Thomson coolers Stirling cycle Stirling engines Stirling coolers Pulse-tube
- 4. What is a « cryo-cooler » A Cryocooler is a standalone cooler, usually of table-top size.
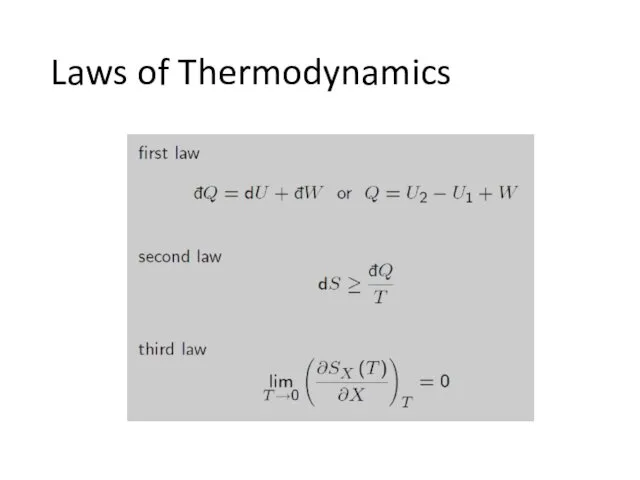
- 5. Laws of Thermodynamics
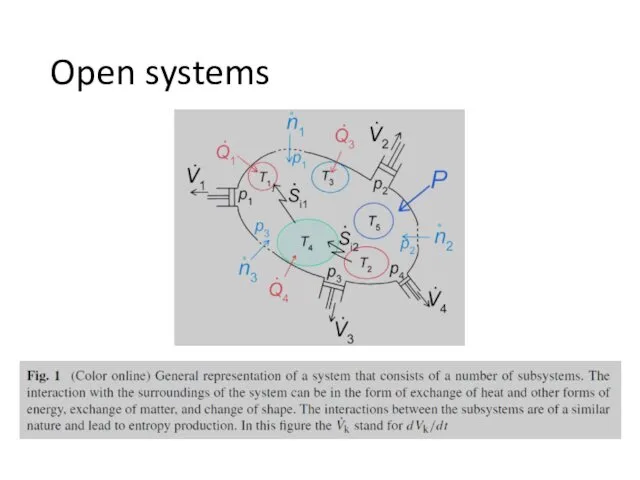
- 6. Open systems
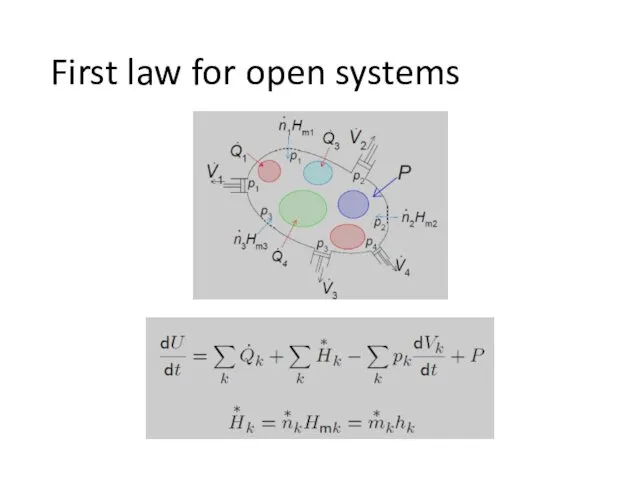
- 7. First law for open systems
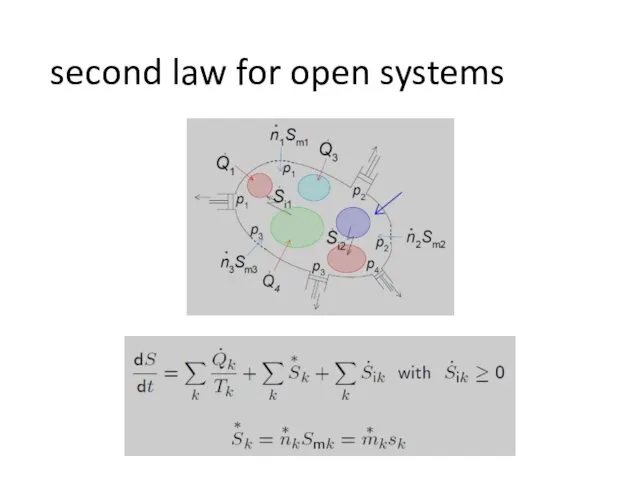
- 8. second law for open systems
- 9. Irreversible processes heat flow over a temperature difference mass flow over a pressure difference diffusion chemical
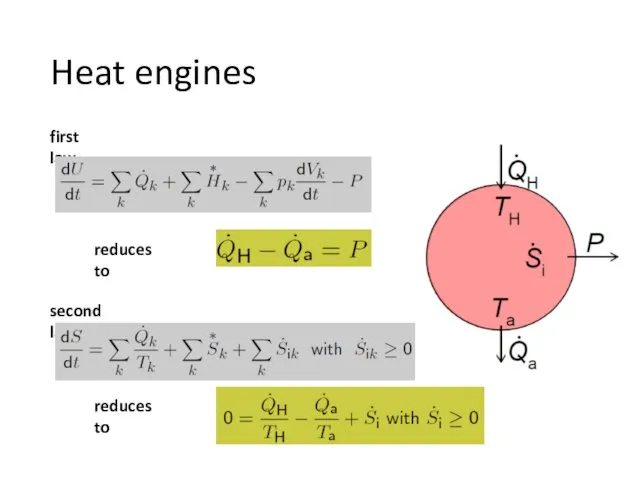
- 10. Heat engines first law reduces to second law reduces to
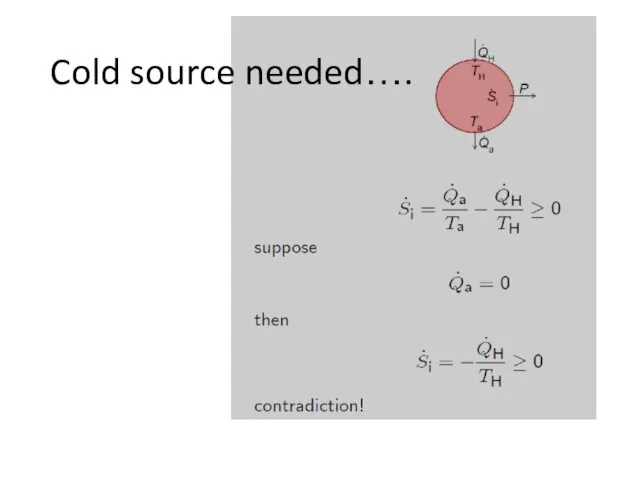
- 11. Cold source needed….
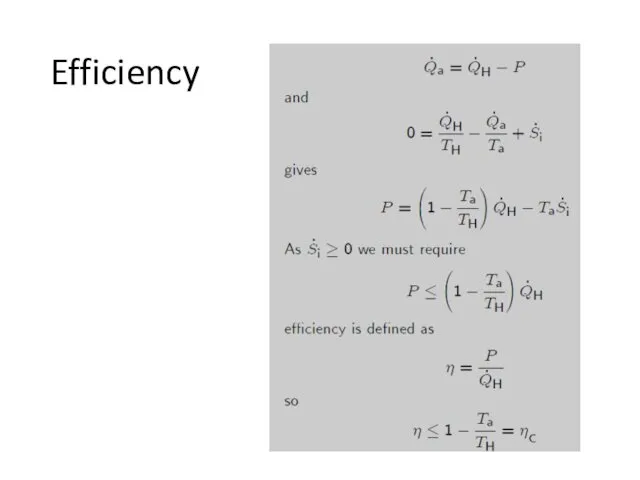
- 12. Efficiency
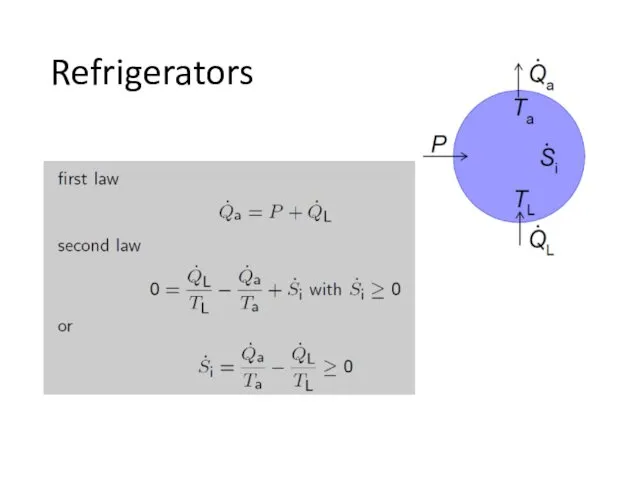
- 13. Refrigerators
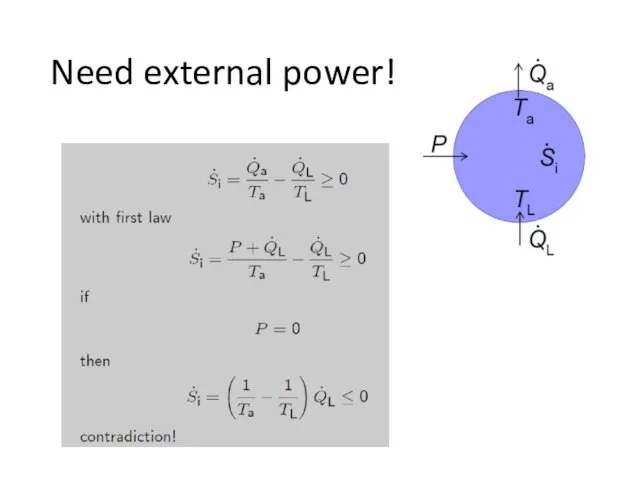
- 14. Need external power!
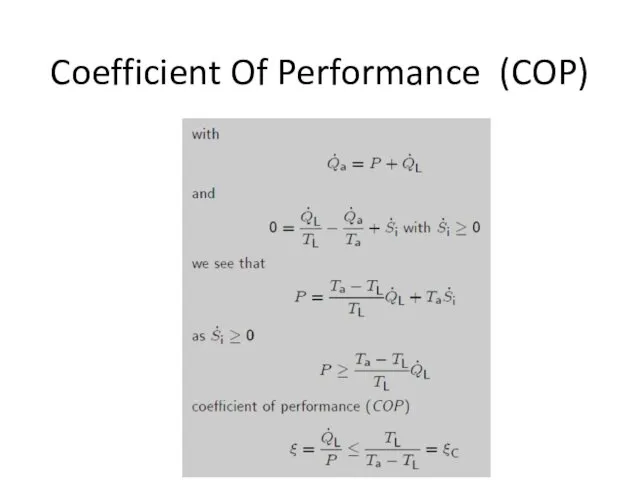
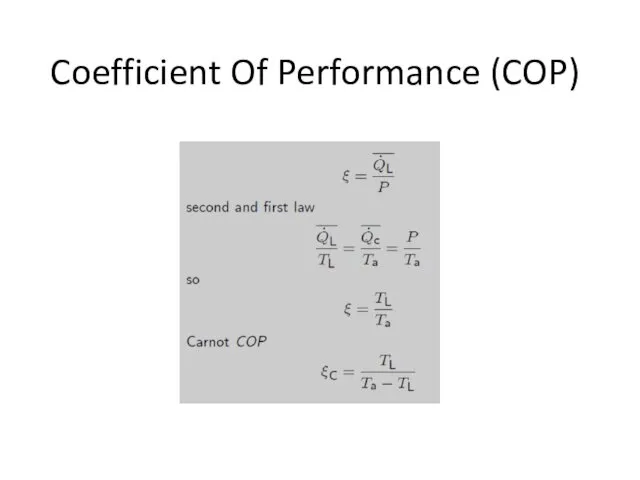
- 15. Coefficient Of Performance (COP)
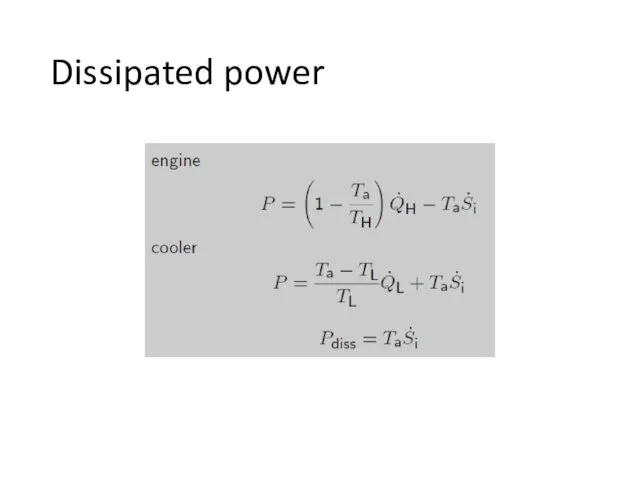
- 16. Dissipated power
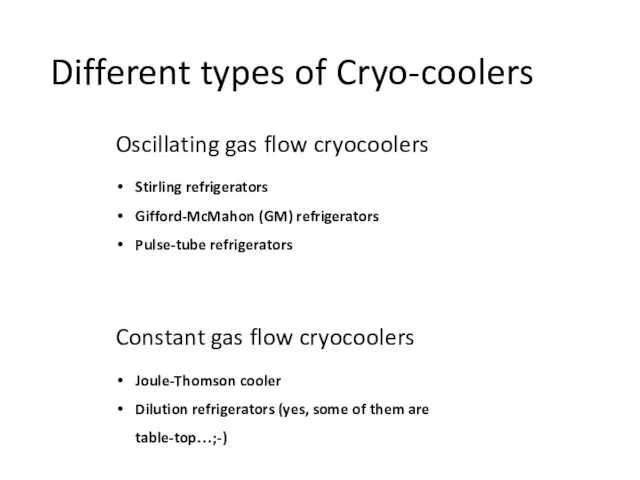
- 17. Different types of Cryo-coolers Oscillating gas flow cryocoolers Stirling refrigerators Gifford-McMahon (GM) refrigerators Pulse-tube refrigerators Constant
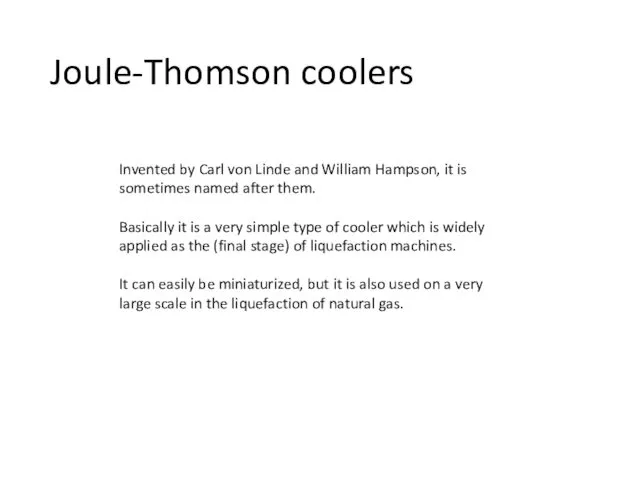
- 18. Joule-Thomson coolers Invented by Carl von Linde and William Hampson, it is sometimes named after them.
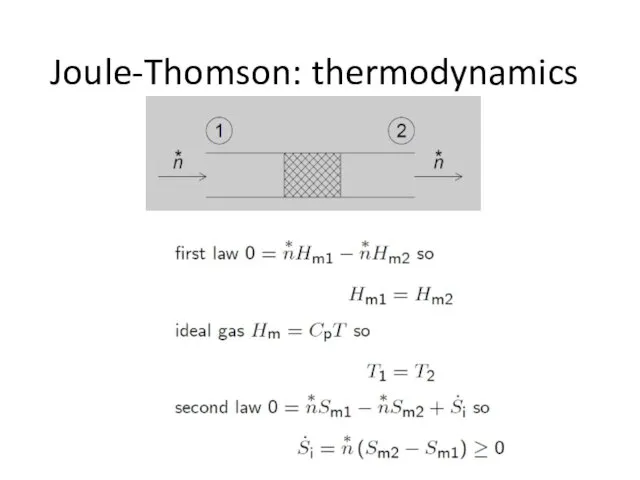
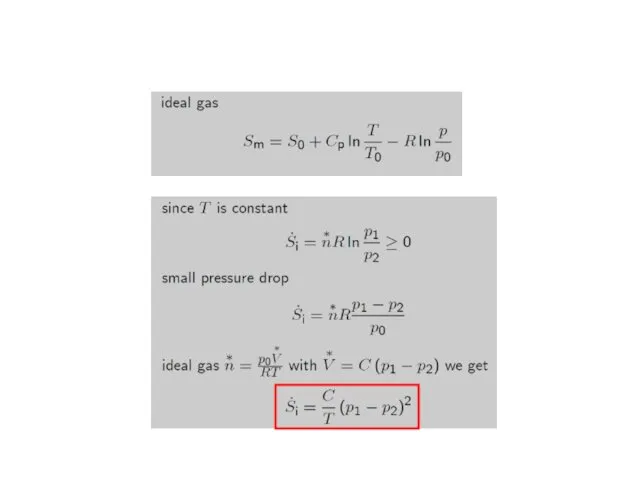
- 19. Joule-Thomson: thermodynamics
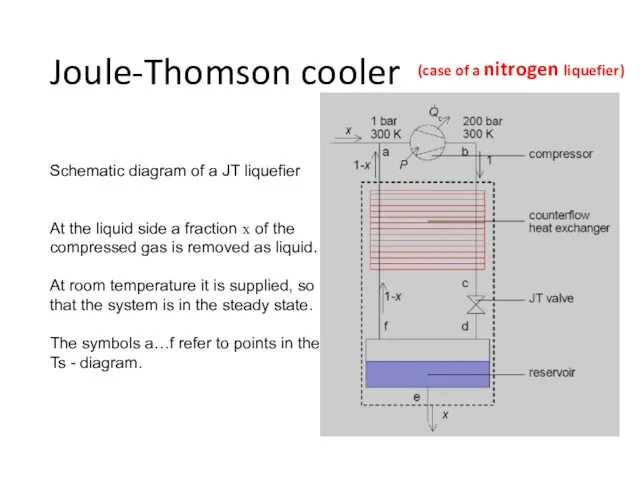
- 21. Joule-Thomson cooler Schematic diagram of a JT liquefier At the liquid side a fraction x of
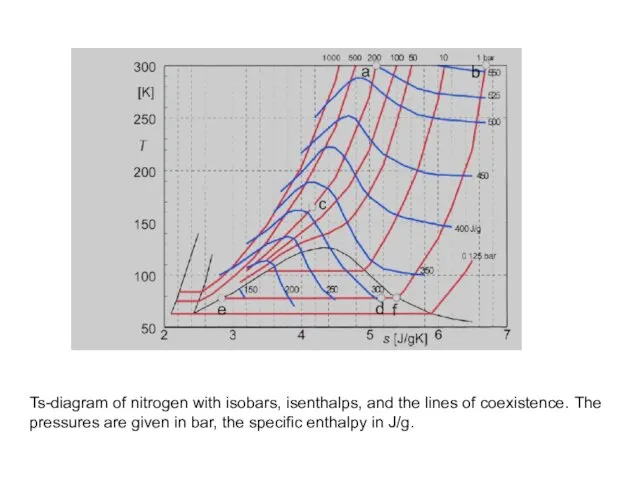
- 22. Ts-diagram of nitrogen with isobars, isenthalps, and the lines of coexistence. The pressures are given in
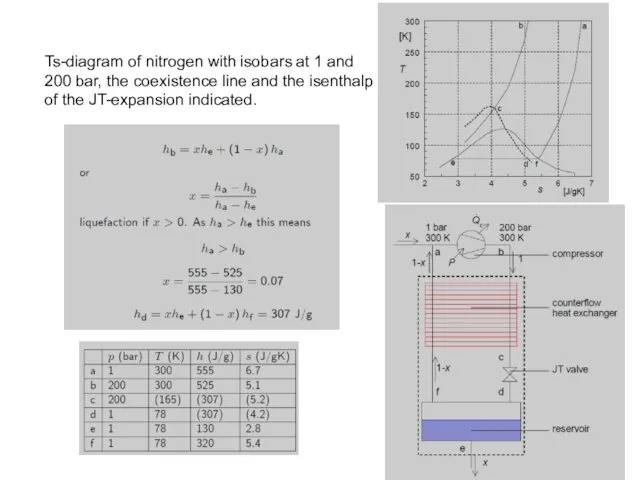
- 23. Ts-diagram of nitrogen with isobars at 1 and 200 bar, the coexistence line and the isenthalp
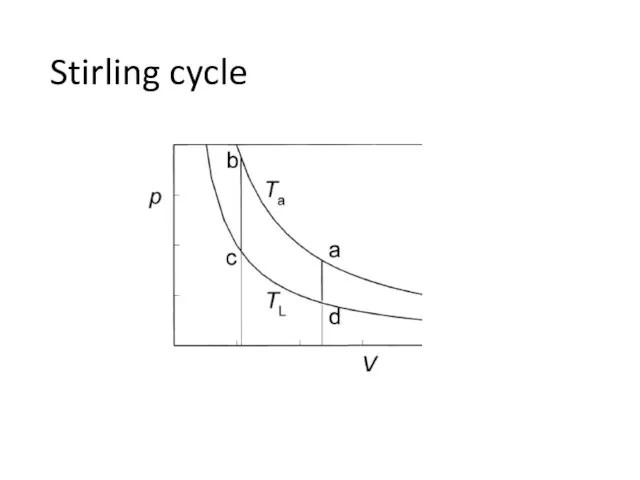
- 24. Stirling cycle
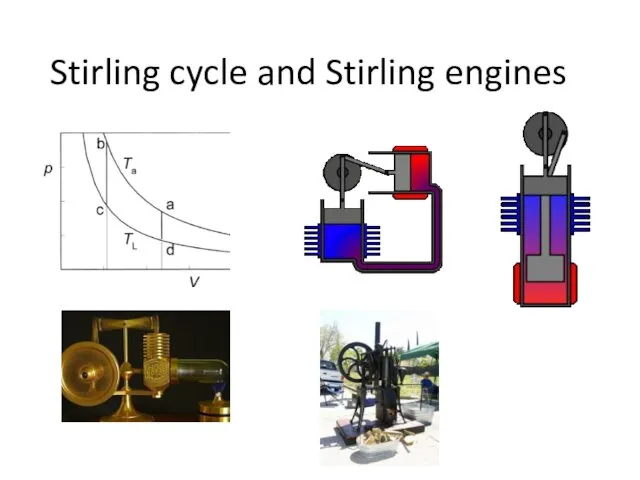
- 25. Stirling cycle and Stirling engines
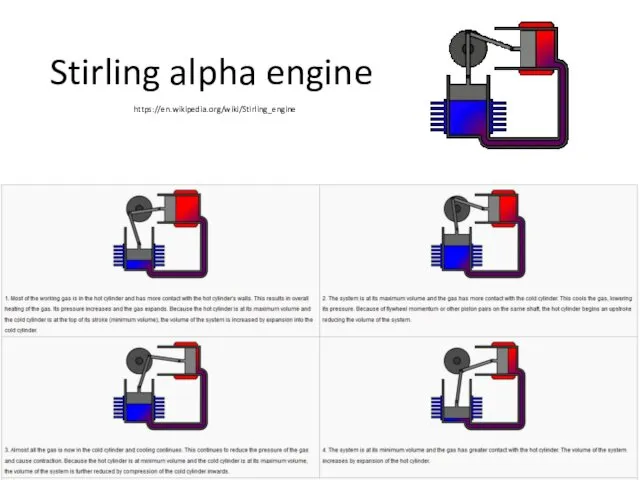
- 26. Stirling alpha engine https://en.wikipedia.org/wiki/Stirling_engine
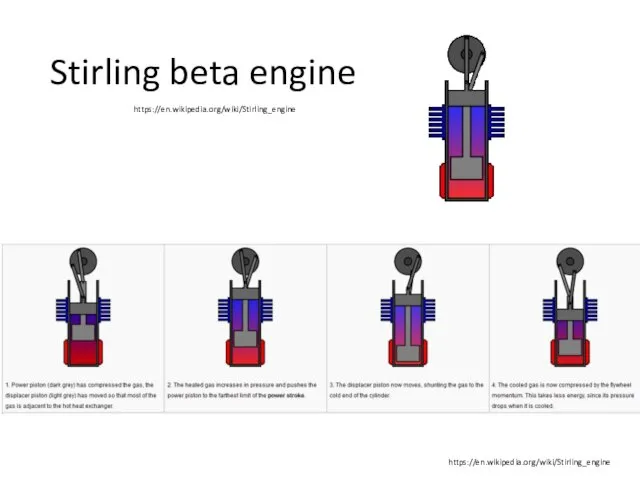
- 27. Stirling beta engine https://en.wikipedia.org/wiki/Stirling_engine https://en.wikipedia.org/wiki/Stirling_engine
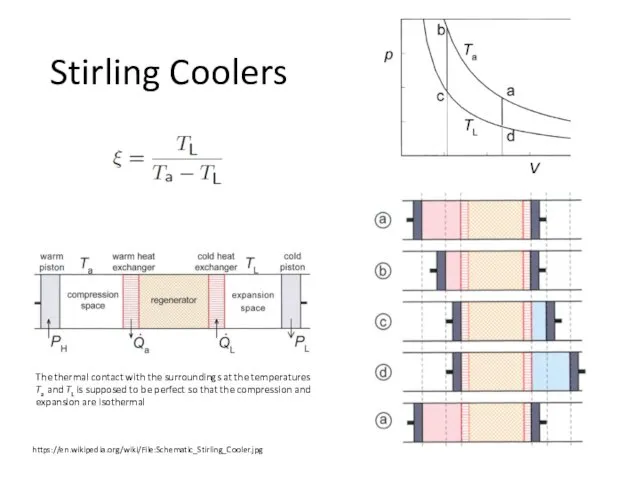
- 28. Stirling Coolers https://en.wikipedia.org/wiki/File:Schematic_Stirling_Cooler.jpg The thermal contact with the surroundings at the temperatures Ta and TL is
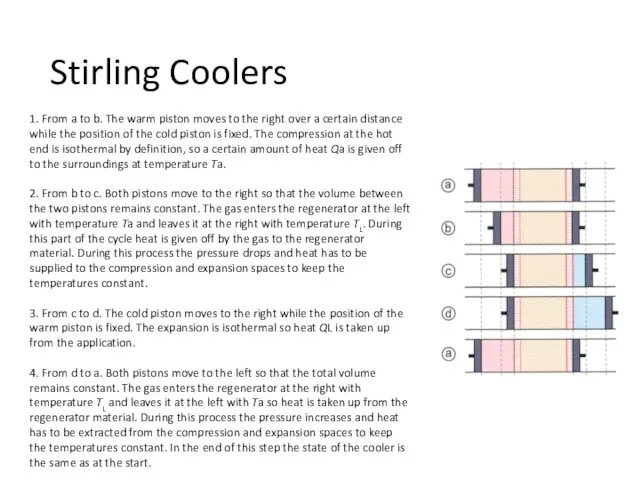
- 29. 1. From a to b. The warm piston moves to the right over a certain distance
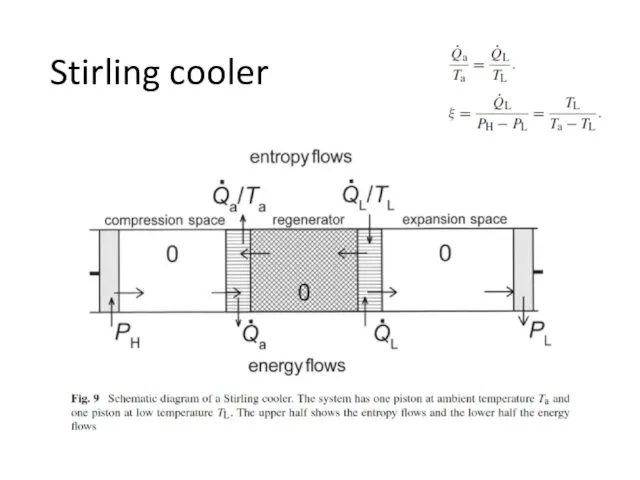
- 30. Stirling cooler
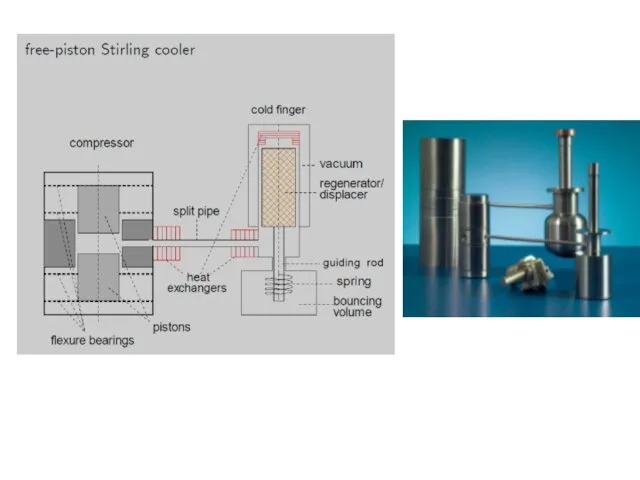
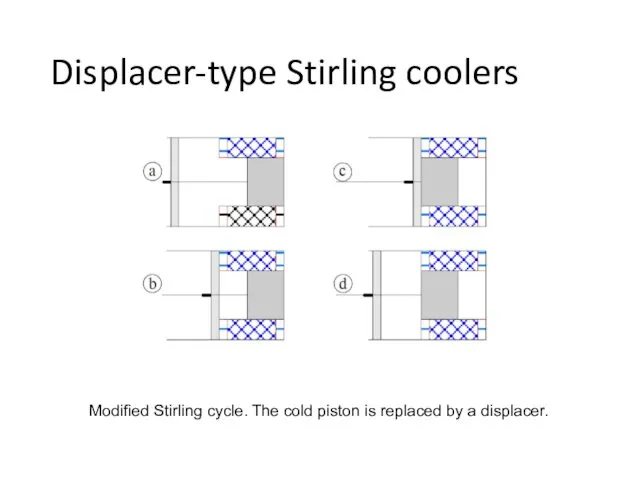
- 32. Displacer-type Stirling coolers Modified Stirling cycle. The cold piston is replaced by a displacer.
- 33. PULSE-TUBE REFRIGERATORS (PTRs)
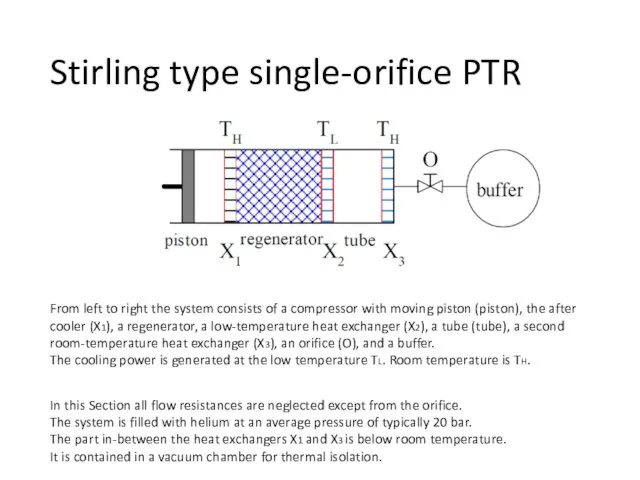
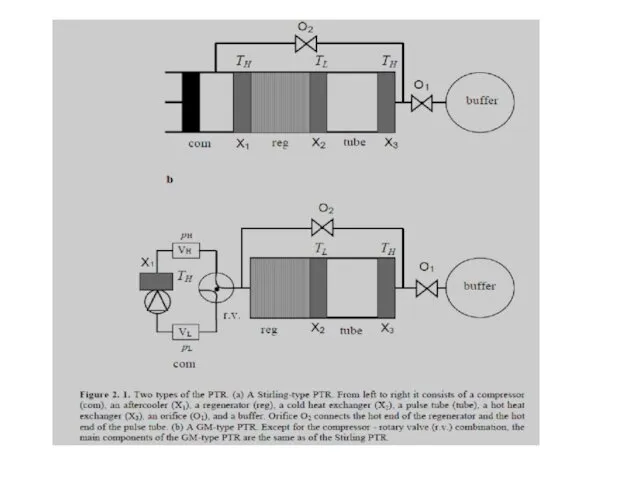
- 34. Stirling type single-orifice PTR From left to right the system consists of a compressor with moving
- 35. Some remarks… The piston moves the gas back and forth and generates a varying pressure in
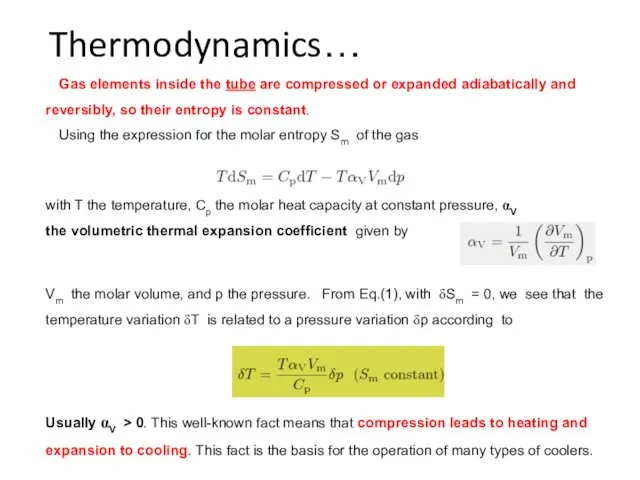
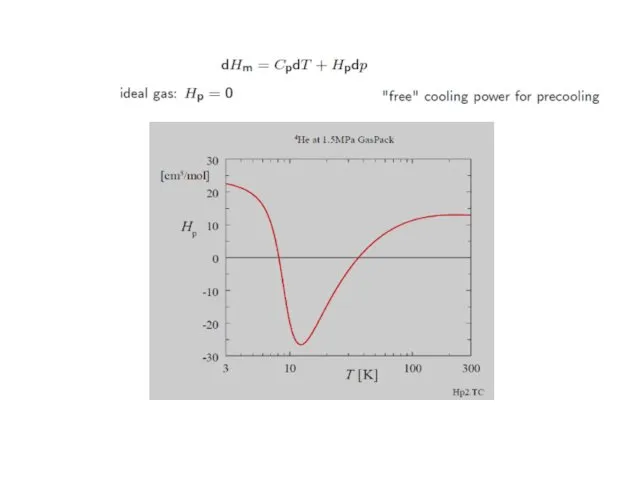
- 36. Thermodynamics… with T the temperature, Cp the molar heat capacity at constant pressure, αV the volumetric
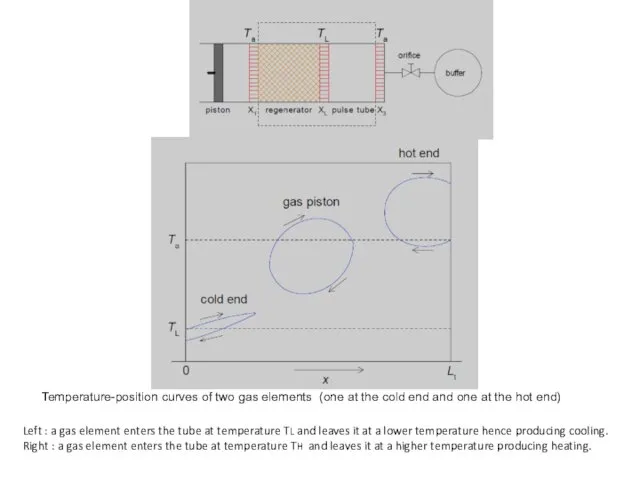
- 37. Left : a gas element enters the tube at temperature TL and leaves it at a
- 38. At the hot end gas flows from the buffer via the orifice into the tube with
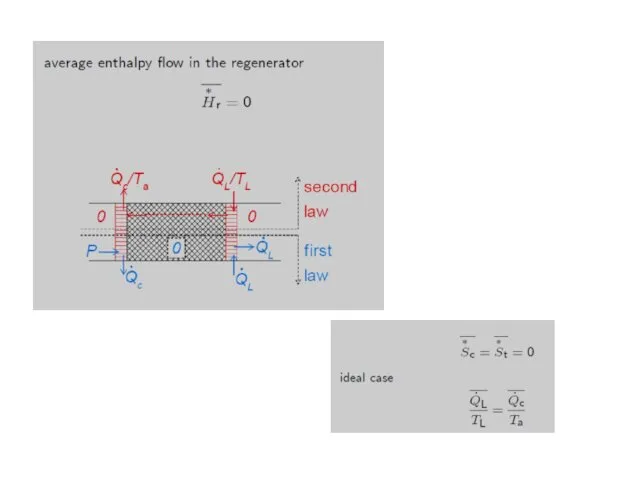
- 39. Ideal regenerators The thermodynamic and hydrodynamic properties of regenerators usually are extremely complicated. In many cases
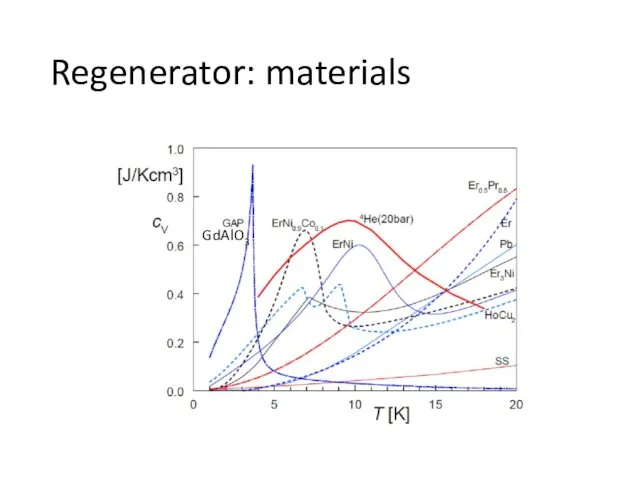
- 40. Regenerator: materials
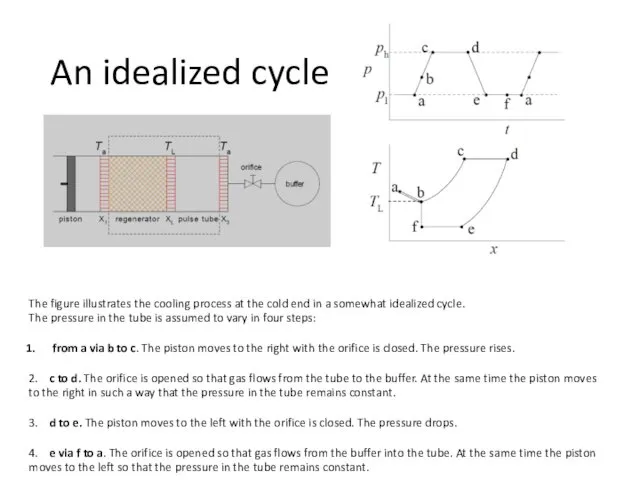
- 41. The figure illustrates the cooling process at the cold end in a somewhat idealized cycle. The
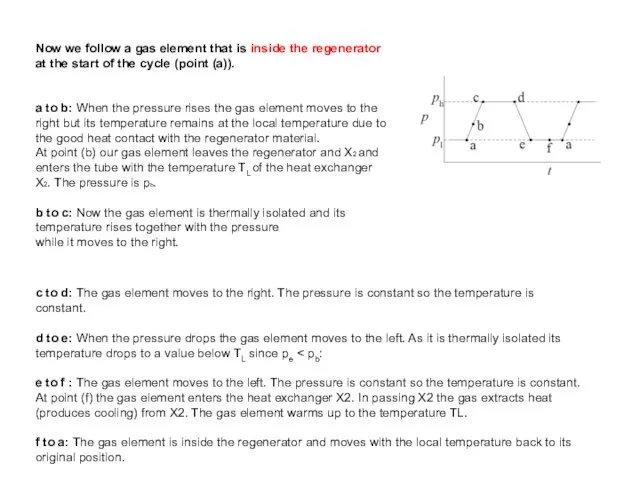
- 42. Now we follow a gas element that is inside the regenerator at the start of the
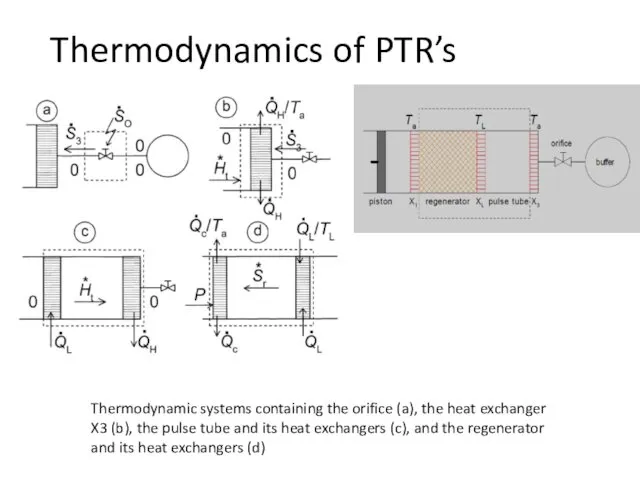
- 43. Thermodynamics of PTR’s Ideal PTR: dissipation only occurs in the orifice
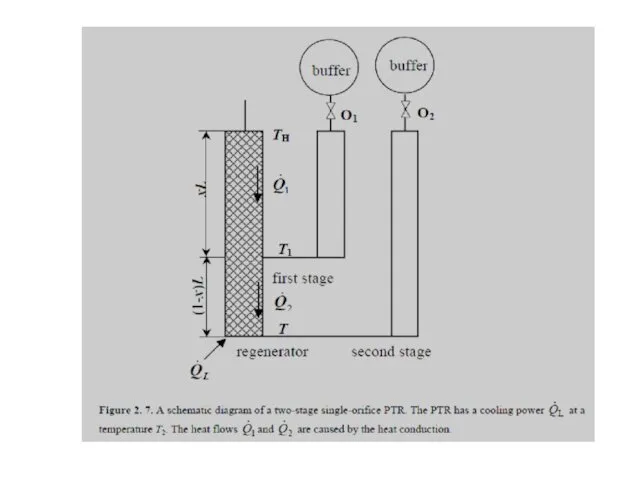
- 44. Thermodynamic systems containing the orifice (a), the heat exchanger X3 (b), the pulse tube and its
- 46. Coefficient Of Performance (COP)
- 47. Pulse-tube refrigerators have their origin in an observation that W. E. Gifford made, while working on
- 48. The PTR has no moving parts in the low-temperature region, and, therefore, has a long lifetime
- 49. The main breakthrough came in 1984, when Mikulin and his co-workers invented the Orifice Pulse Tube
- 50. PhD Thesis Low-temperature cryocooling / by Irina Tanaeva. - Eindhoven : Technische Universiteit Eindhoven, 2004. –
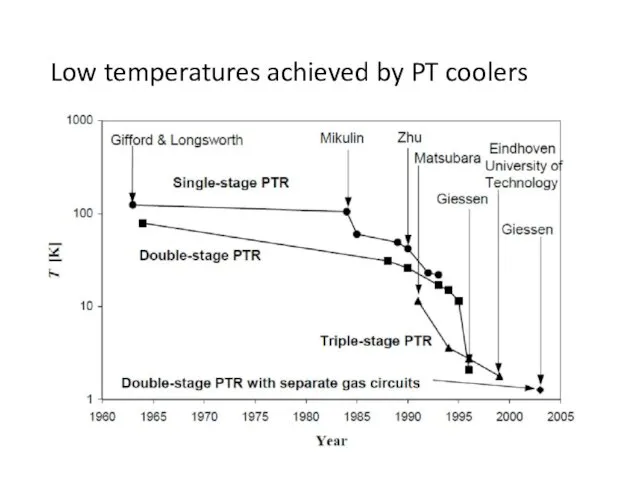
- 51. Low temperatures achieved by PT coolers
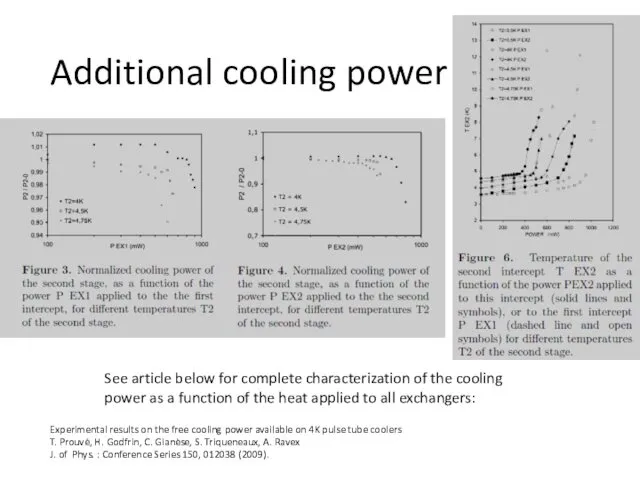
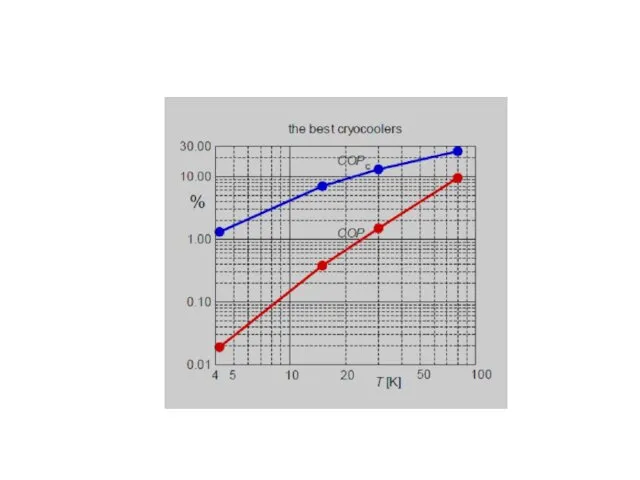
- 54. Additional cooling power [5] Experimental results on the free cooling power available on 4K pulse tube
- 55. Additional cooling power Experimental results on the free cooling power available on 4K pulse tube coolers
- 57. Commercial pulse-tubes
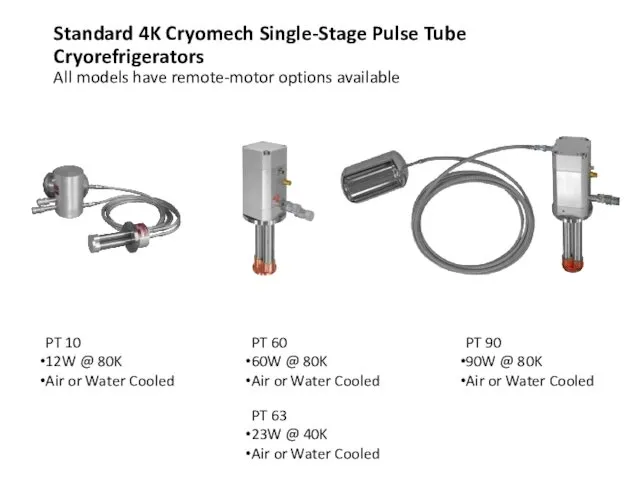
- 58. PT 10 12W @ 80K Air or Water Cooled PT 60 60W @ 80K Air or
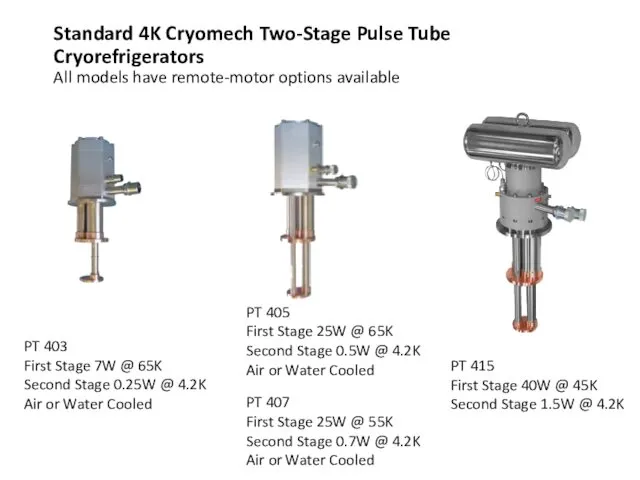
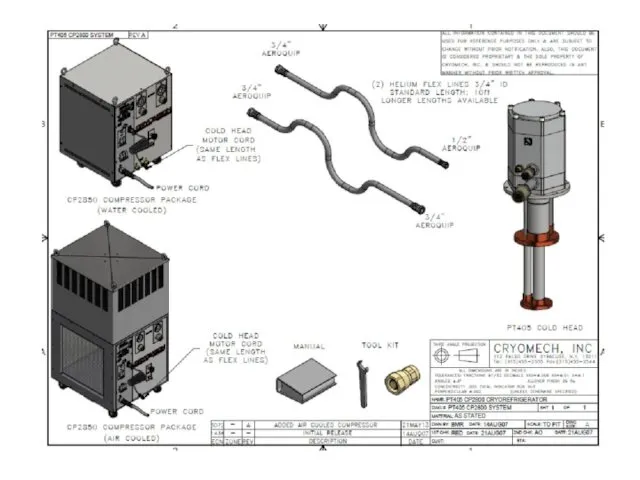
- 59. Standard 4K Cryomech Two-Stage Pulse Tube Cryorefrigerators All models have remote-motor options available PT 403 First
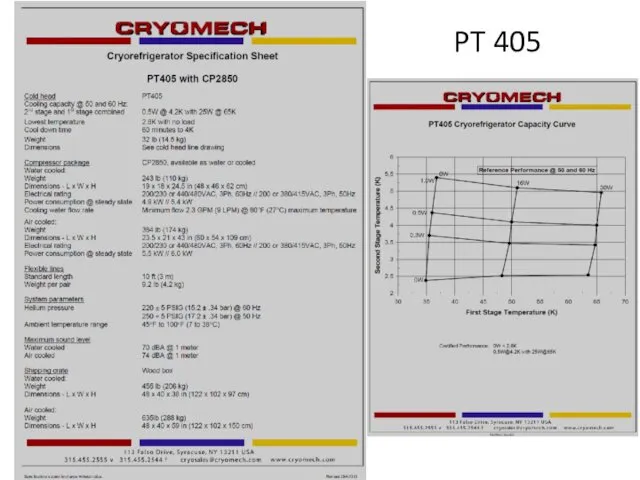
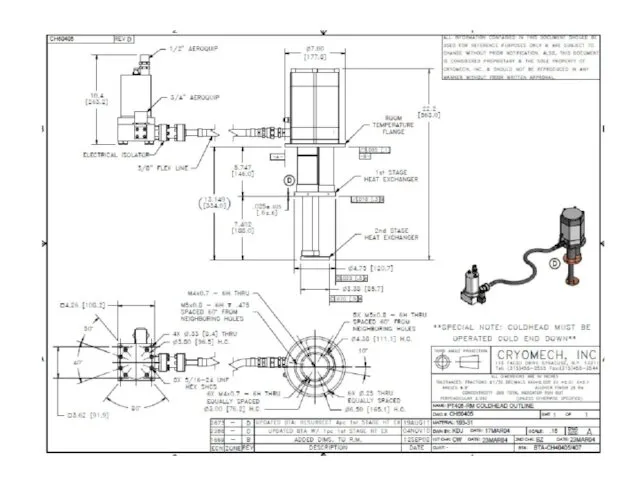
- 60. PT 405
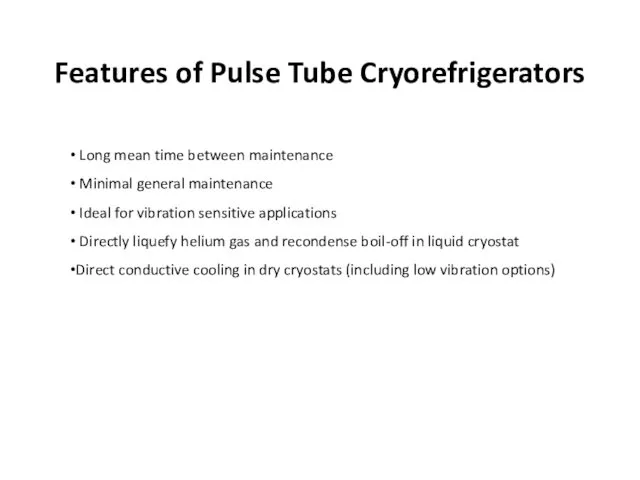
- 64. Features of Pulse Tube Cryorefrigerators Long mean time between maintenance Minimal general maintenance Ideal for vibration
- 65. Liquid Helium Plants and Recovery Systems Liquefaction rates from 6-60 liters per day
- 66. Helium Reliquefiers
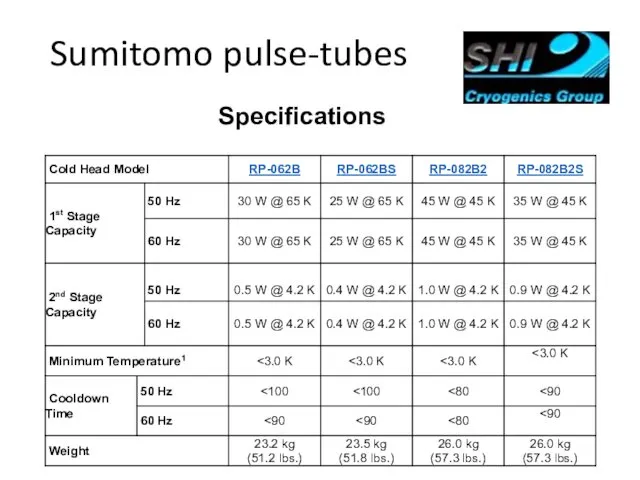
- 67. Sumitomo pulse-tubes Specifications
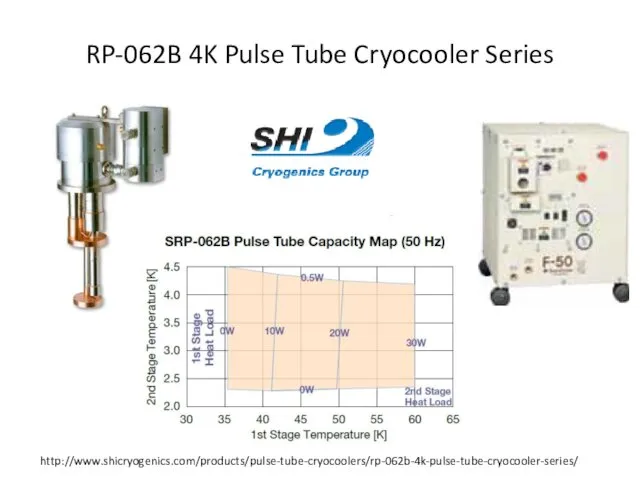
- 68. RP-062B 4K Pulse Tube Cryocooler Series http://www.shicryogenics.com/products/pulse-tube-cryocoolers/rp-062b-4k-pulse-tube-cryocooler-series/
- 69. Other manufacturers Advanced Research Systems (ARS) http://www.arscryo.com/ Thales Cryogenics http://www.thales-cryogenics.com
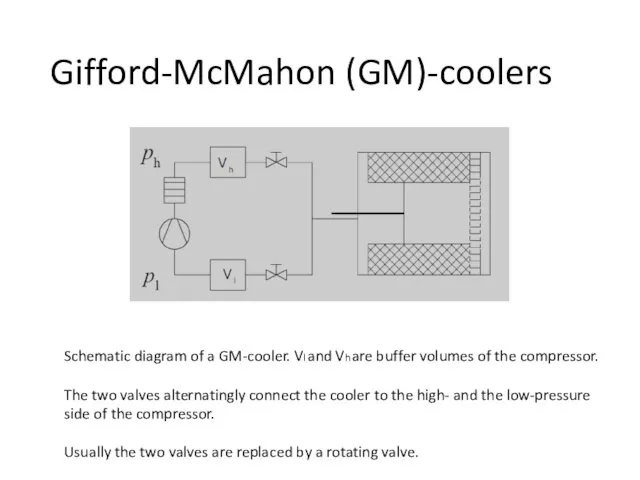
- 70. Gifford-McMahon (GM)-coolers Schematic diagram of a GM-cooler. Vl and Vh are buffer volumes of the compressor.
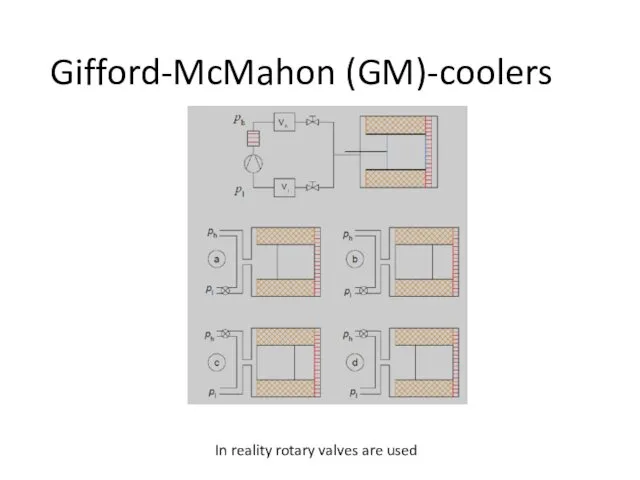
- 71. Gifford-McMahon (GM)-coolers In reality rotary valves are used
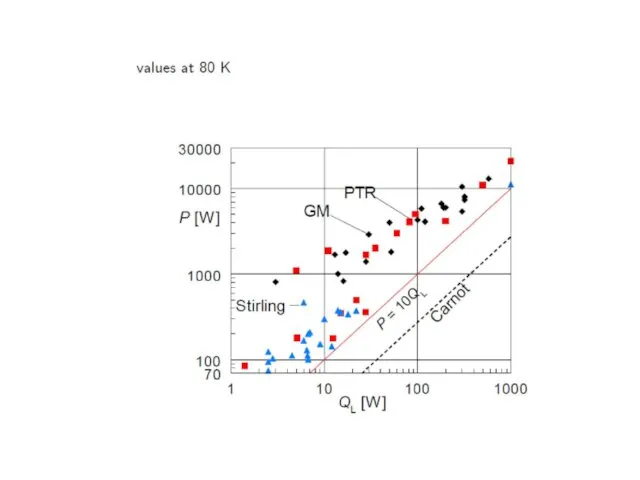
- 72. Gifford-McMahon (GM)-coolers
- 76. Скачать презентацию










![Additional cooling power [5] Experimental results on the free cooling](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/88952/slide-53.jpg)





 Фотоефект та його застосування
Фотоефект та його застосування Цифровые сигналы
Цифровые сигналы Волновая оптика (11 класс)
Волновая оптика (11 класс) Законы Ньютона. 10 Класс
Законы Ньютона. 10 Класс Давление твёрдых тел. Вычисление давления на опору
Давление твёрдых тел. Вычисление давления на опору Электрический ток в полупроводниках
Электрический ток в полупроводниках Оптические явления. Урок для 8 класса
Оптические явления. Урок для 8 класса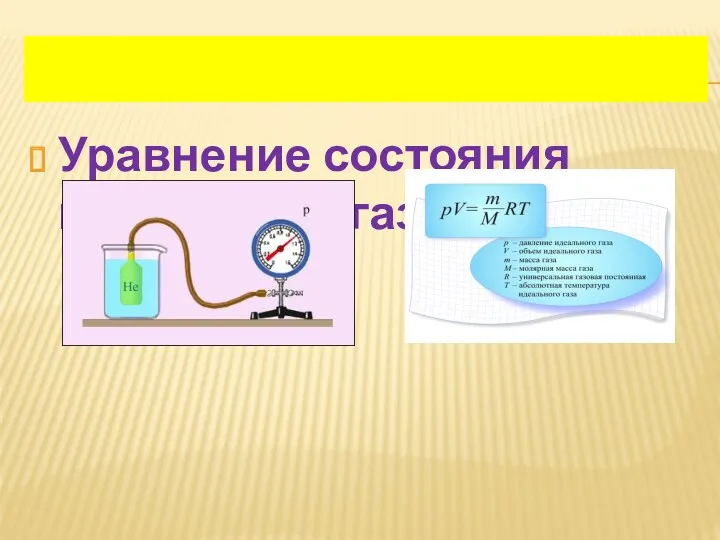 Уравнение состояния идеального газа
Уравнение состояния идеального газа Кинематика движения материальной точки
Кинематика движения материальной точки Параллельное соединение проводников
Параллельное соединение проводников Маневренность (Управляемость корабля)
Маневренность (Управляемость корабля) Расчёт основных характеристик цепей переменного тока
Расчёт основных характеристик цепей переменного тока Линза. Применение линз. Виды линз. Построение изображения в линзах
Линза. Применение линз. Виды линз. Построение изображения в линзах Инфракрасная спектроскопия
Инфракрасная спектроскопия Презентация для будущих первоклассников на тему Я - наблюдатель
Презентация для будущих первоклассников на тему Я - наблюдатель Небольшое небесное тело комета
Небольшое небесное тело комета Классификация и технические характеристики военных радиостанций. (Лекция 7)
Классификация и технические характеристики военных радиостанций. (Лекция 7) Презентация к урокупо теме Третий закон Ньютона, 9 кл.
Презентация к урокупо теме Третий закон Ньютона, 9 кл. Введение в динамику системы. Масса. Центр масс. Моменты инерции
Введение в динамику системы. Масса. Центр масс. Моменты инерции Дисциплина Прикладная механика. Основные понятия и определения
Дисциплина Прикладная механика. Основные понятия и определения Работа силы. 10 класс
Работа силы. 10 класс Знакомство с бытовой швейной машиной
Знакомство с бытовой швейной машиной Шкала электромагнитных волн
Шкала электромагнитных волн Робот-исследователь, способный измерять некоторые физические факторы среды
Робот-исследователь, способный измерять некоторые физические факторы среды University physics. Forces review of basic concepts
University physics. Forces review of basic concepts Магнитное поле в веществе. Уравнения Максвелла
Магнитное поле в веществе. Уравнения Максвелла Кран машиниста поезда, с дистанционным управлением №130
Кран машиниста поезда, с дистанционным управлением №130 8 класс
8 класс