Содержание
- 2. Chapter I 1.1 nuclear Structure 1.2 Some properties of nuclei 1.3 Size of nuclei 1.4 Nuclear
- 3. Chapter III Nuclear Force 3.1 Short Range 3.2 Repulsion core 3.3 Charge dependent 3.4 Semi empirical
- 4. Nuclear Structure Atoms consist of electrons in orbit about a central nucleus. As we have seen
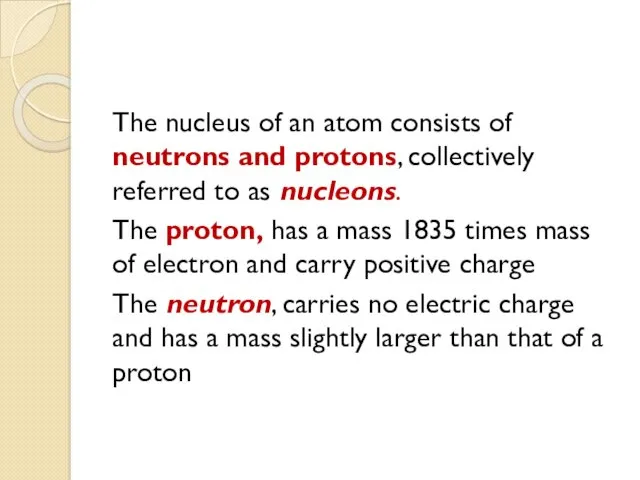
- 5. The nucleus of an atom consists of neutrons and protons, collectively referred to as nucleons. The
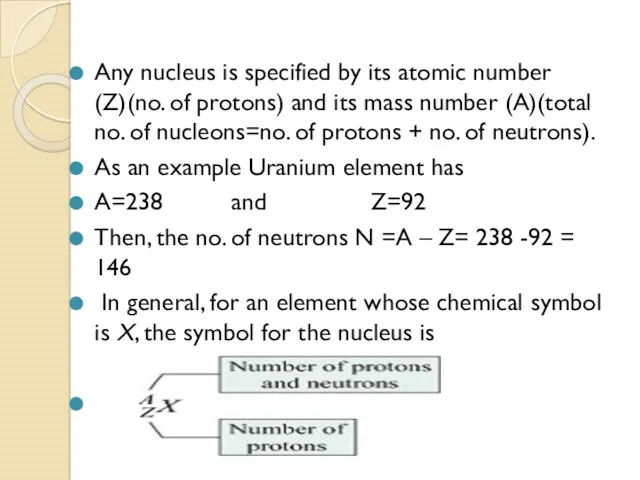
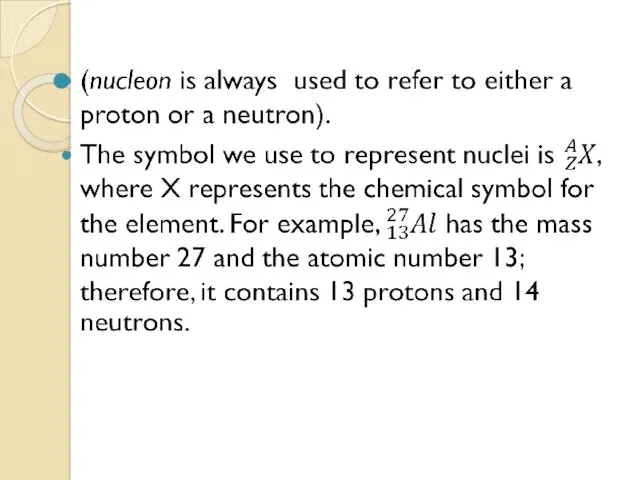
- 6. Any nucleus is specified by its atomic number (Z)(no. of protons) and its mass number (A)(total
- 7. Materials are classified into :
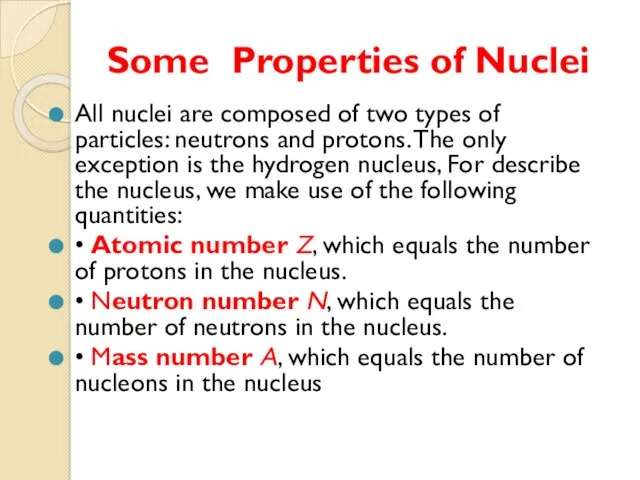
- 9. Some Properties of Nuclei All nuclei are composed of two types of particles: neutrons and protons.
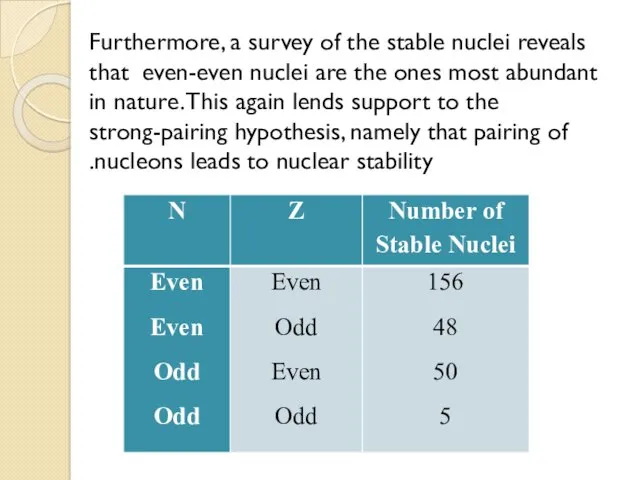
- 10. Furthermore, a survey of the stable nuclei reveals that even-even nuclei are the ones most abundant
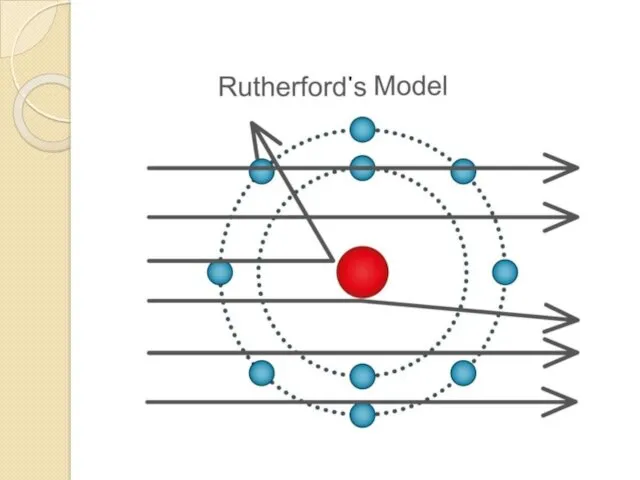
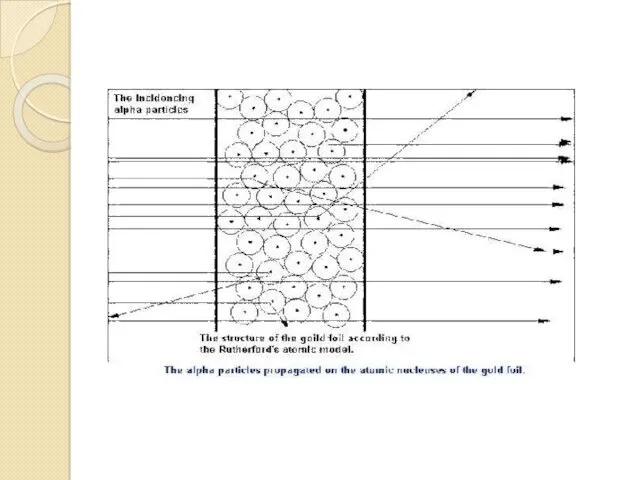
- 11. Rutherford's experiment Principle of Rutherford's experiment. By bombarding a very thin gold foil with alpha particles,
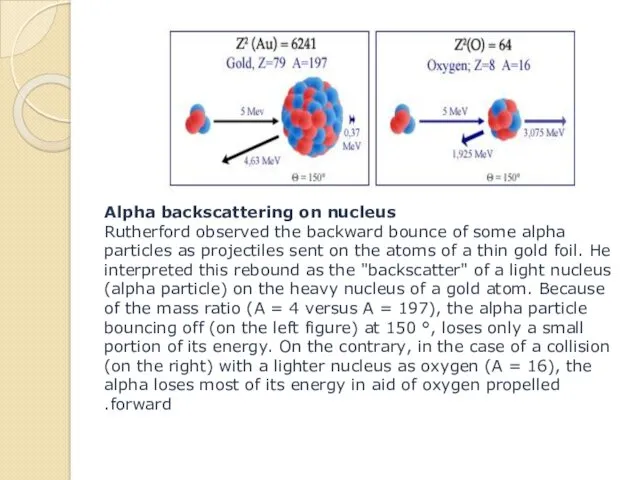
- 14. Alpha backscattering on nucleus Rutherford observed the backward bounce of some alpha particles as projectiles sent
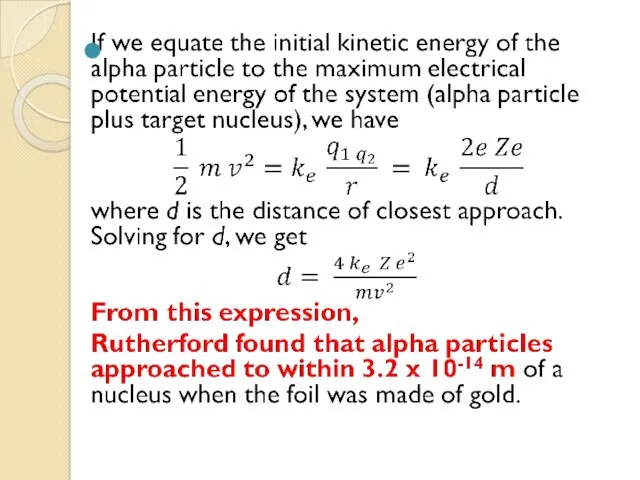
- 15. The Size of Nuclei Rutherford found an expression for how close an alpha particle moving directly
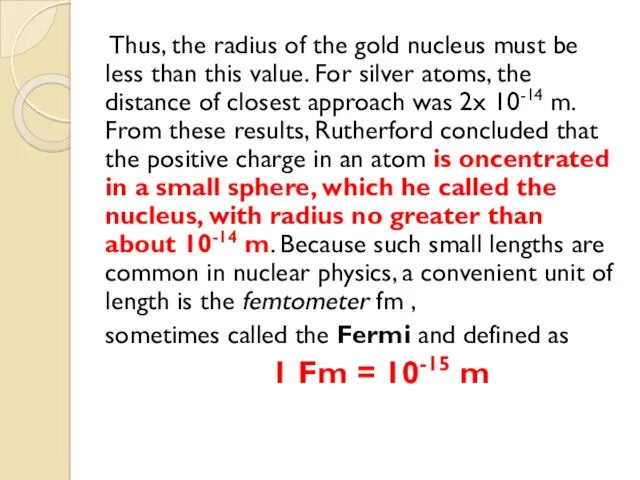
- 17. Thus, the radius of the gold nucleus must be less than this value. For silver atoms,
- 18. Calculation of Nuclear Radius The nuclear radius (R) is considered to be one of the basic
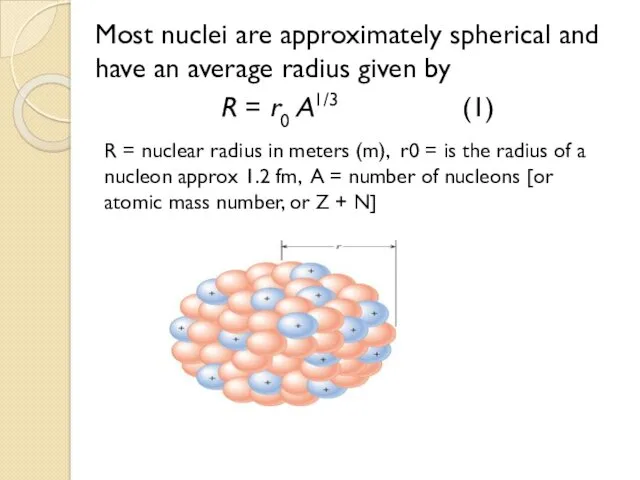
- 19. Most nuclei are approximately spherical and have an average radius given by R = r0 A1/3
- 20. where A is the total number of nucleons and r0 is a constant equal to 1.2
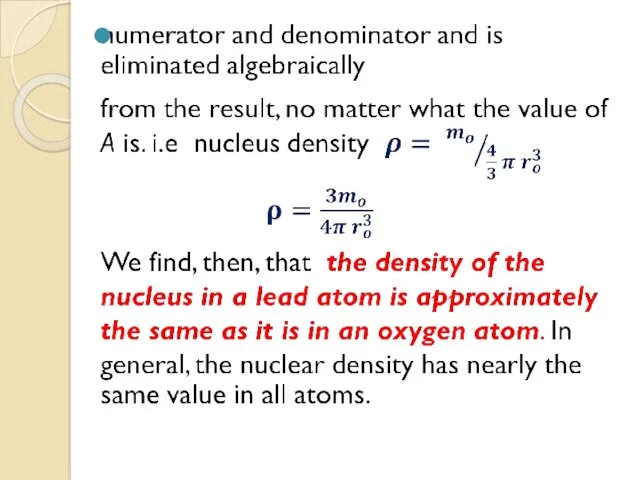
- 21. It is well known that lead and oxygen contain different atoms and that the density of
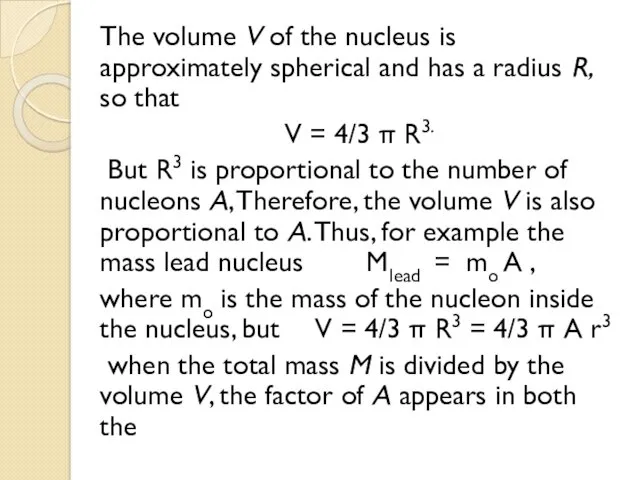
- 22. The volume V of the nucleus is approximately spherical and has a radius R, so that
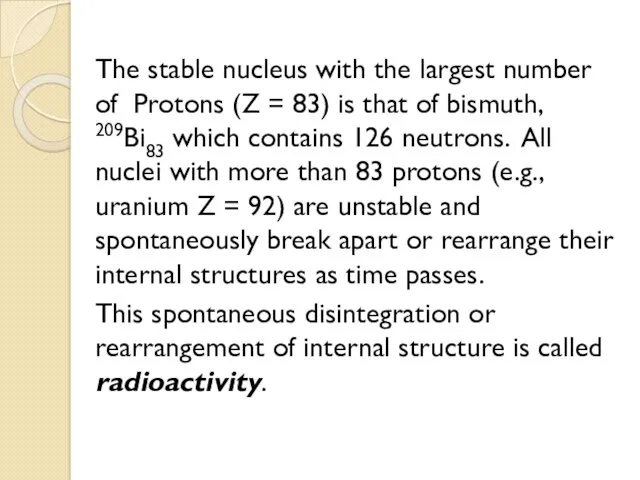
- 24. The stable nucleus with the largest number of Protons (Z = 83) is that of bismuth,
- 25. Two positive charges that are as close together as they are in a nucleus repel one
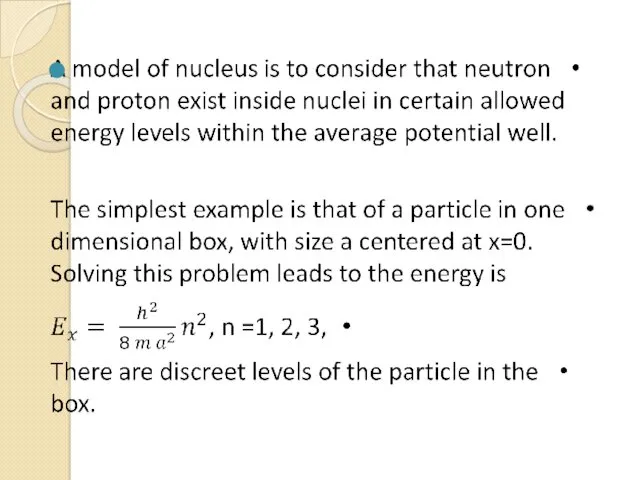
- 26. so a different type of force must hold the nucleus together. This force is the strong
- 27. Many features of the strong nuclear force are well known. For example, it is almost independent
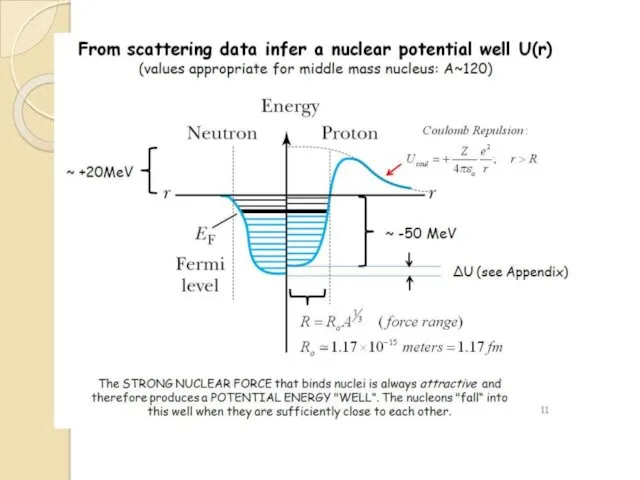
- 28. The limited range of action of the strong nuclear force plays an important role in the
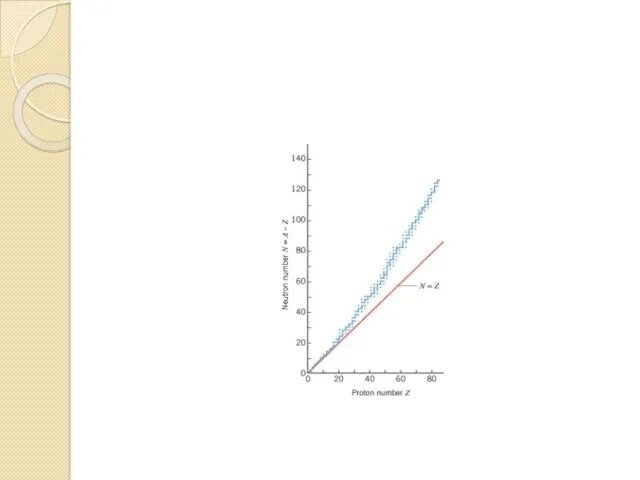
- 29. As in Fig. shows a plot of N versus Z for naturally occurring elements that have
- 31. As more and more protons occur in a nucleus, there comes a point when a balance
- 32. All nuclei with more than 83 protons (e.g., uranium, Z=92) are unstable and spontaneously break apart
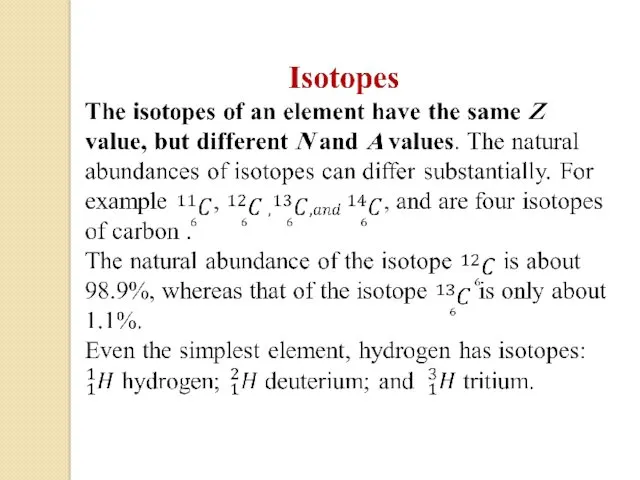
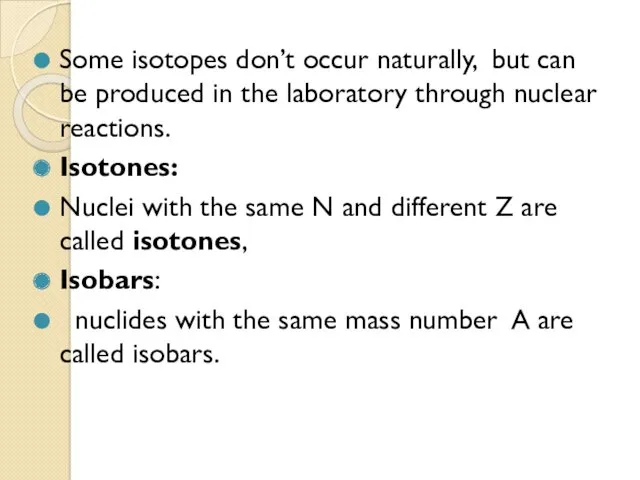
- 34. Some isotopes don’t occur naturally, but can be produced in the laboratory through nuclear reactions. Isotones:
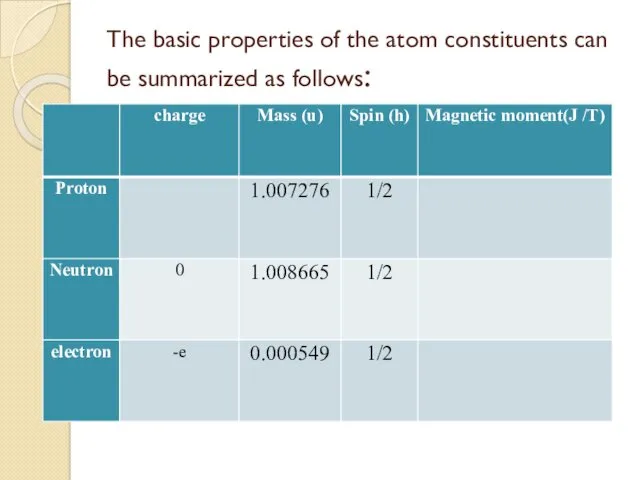
- 35. The basic properties of the atom constituents can be summarized as follows:
- 36. Spin: Each of the atomic constituents has spin ½ h and is an example of what
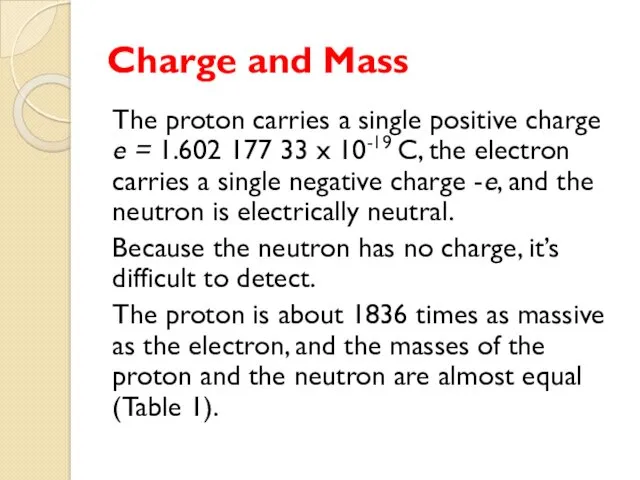
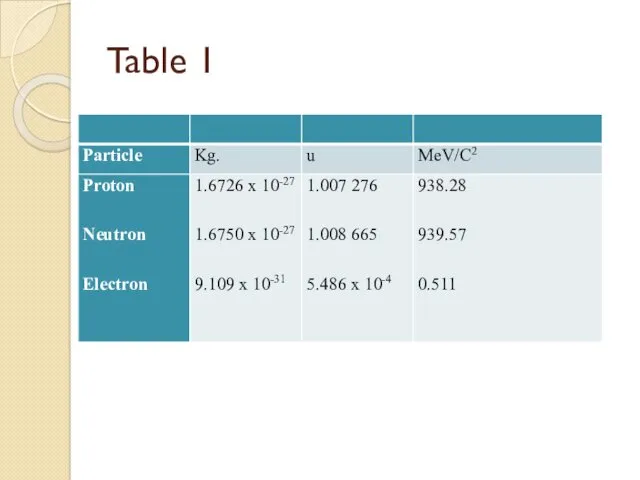
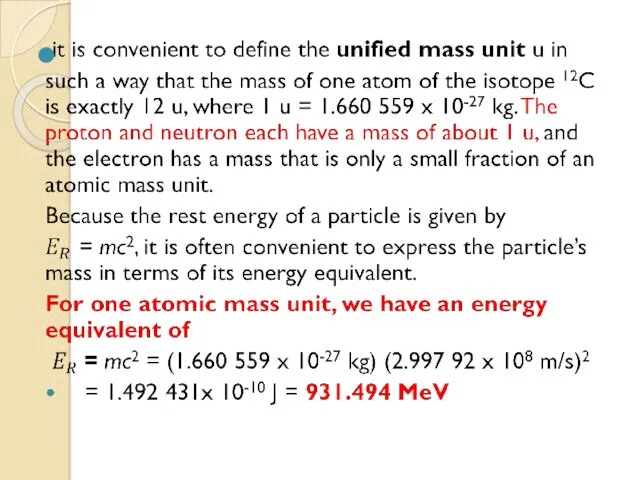
- 37. Charge and Mass The proton carries a single positive charge e = 1.602 177 33 x
- 38. Table 1
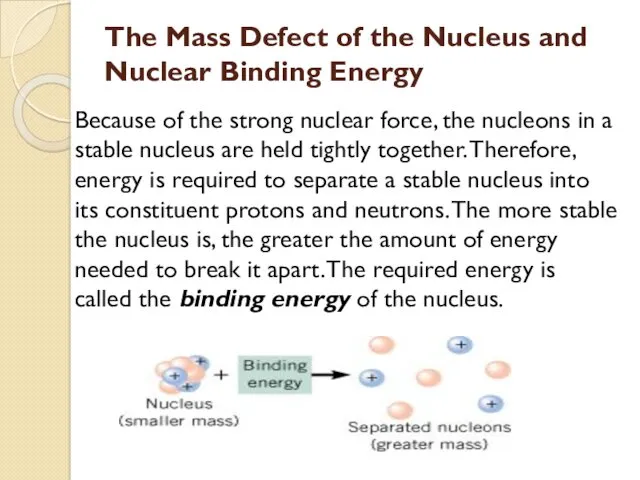
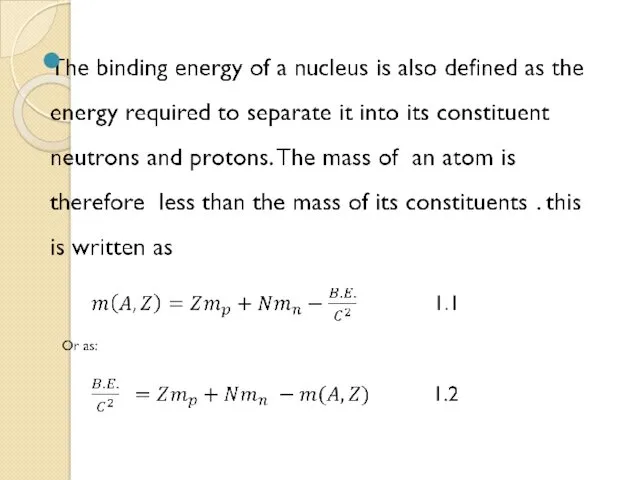
- 40. The Mass Defect of the Nucleus and Nuclear Binding Energy Because of the strong nuclear force,
- 41. Energy, called the binding energy, must be supplied to break the nucleus apart into its constituent
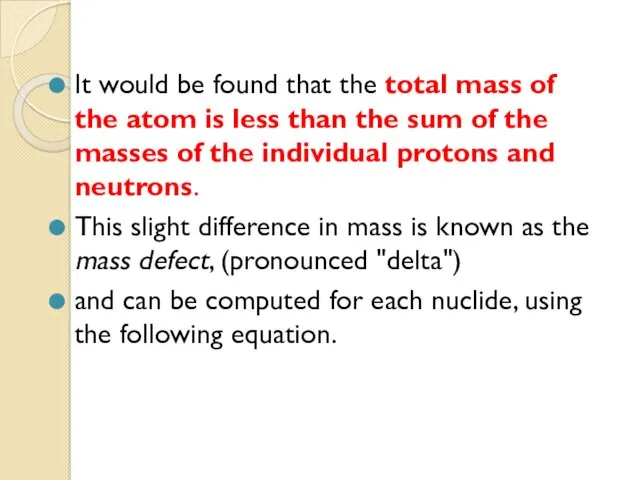
- 42. It would be found that the total mass of the atom is less than the sum
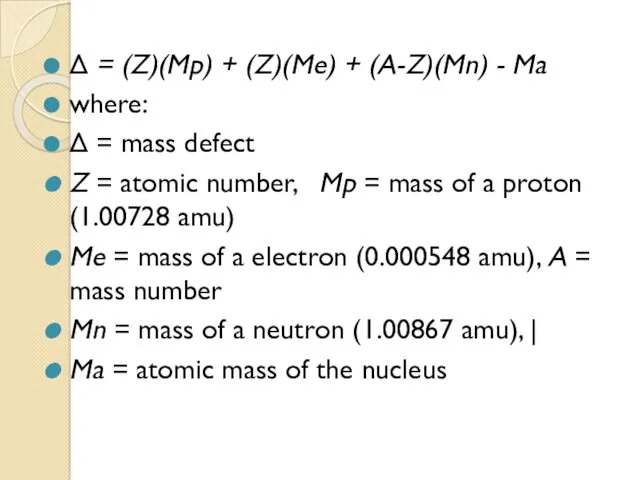
- 43. Δ = (Z)(Mp) + (Z)(Me) + (A-Z)(Mn) - Ma where: Δ = mass defect Z =
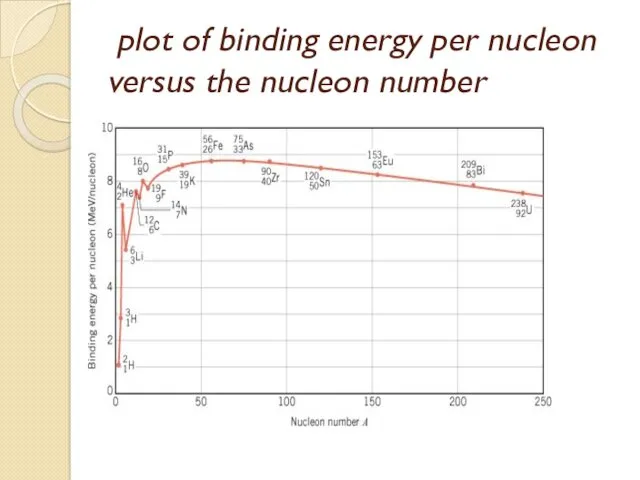
- 45. To see how the nuclear binding energy varies from nucleus to nucleus, it is necessary to
- 46. rapidly for nuclei with small masses and reaches a maximum of approximately 8.7 MeV/ nucleon for
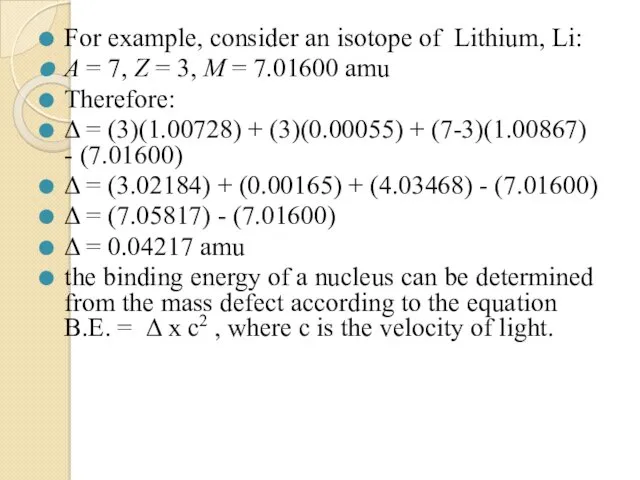
- 47. For example, consider an isotope of Lithium, Li: A = 7, Z = 3, M =
- 48. plot of binding energy per nucleon versus the nucleon number
- 52. Radioactivity When an unstable or radioactive nucleus disintegrates spontaneously, certain kinds of particles and/or high energy
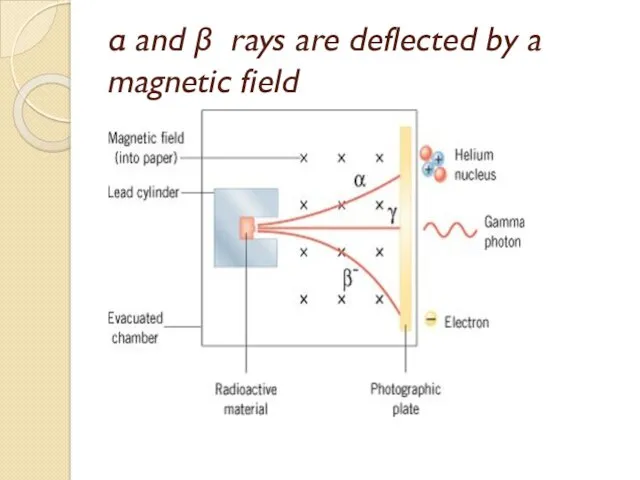
- 53. α and β rays are deflected by a magnetic field
- 54. Alpha particles (α) (+ve charge, helium nuclei He). Beta particles (β) (-ve charge, electrons). Gamma ray
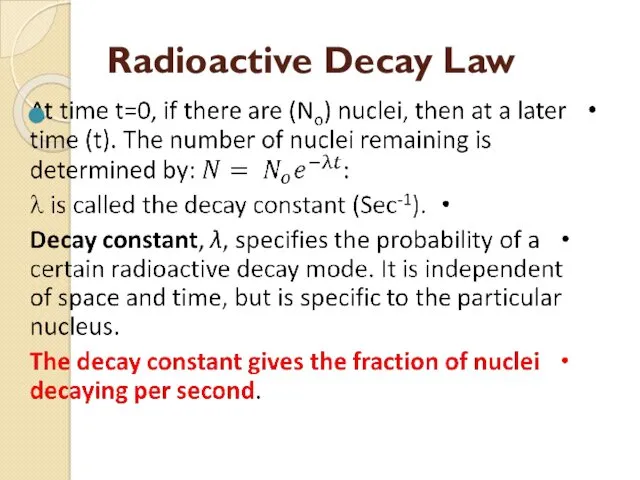
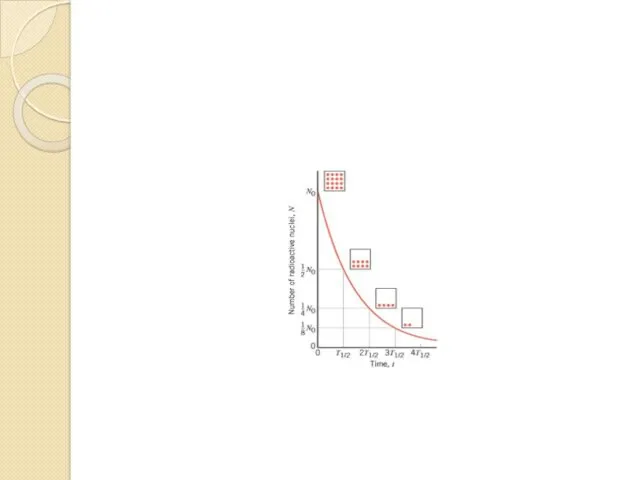
- 55. Radioactive Decay Law
- 56. Half-life (τ)
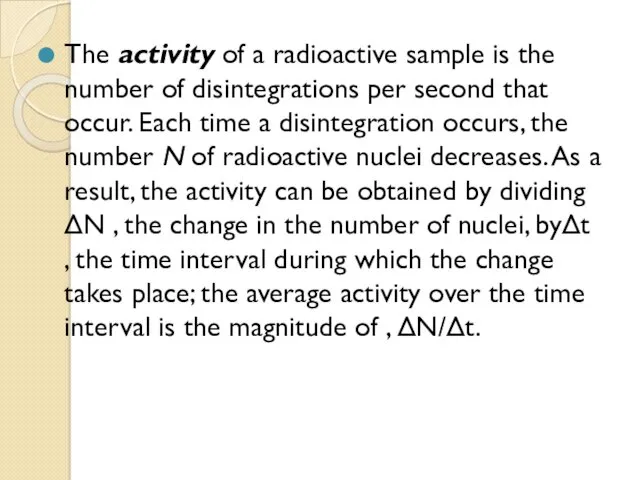
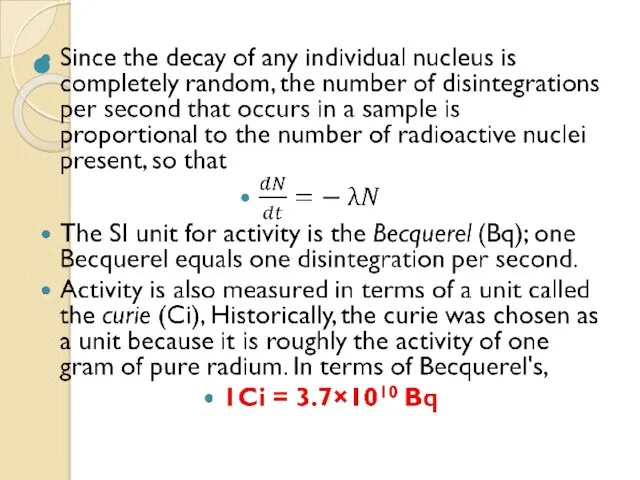
- 58. The activity of a radioactive sample is the number of disintegrations per second that occur. Each
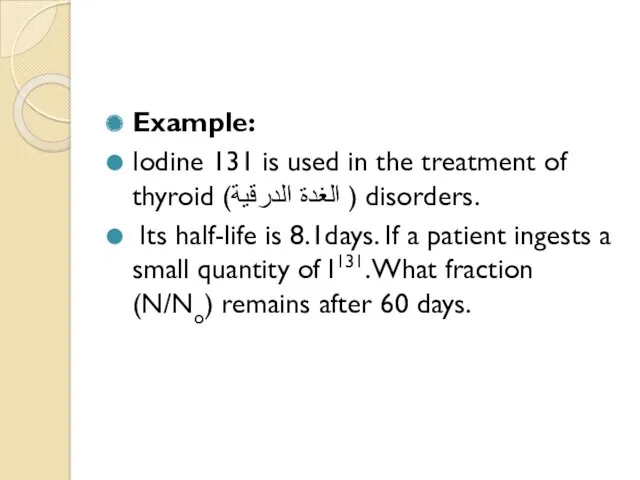
- 60. Example: Iodine 131 is used in the treatment of thyroid (الغدة الدرقية ) disorders. Its half-life
- 61. Problems After 24 hours the radioactivity of a nuclide is one-eighth times its original level. What
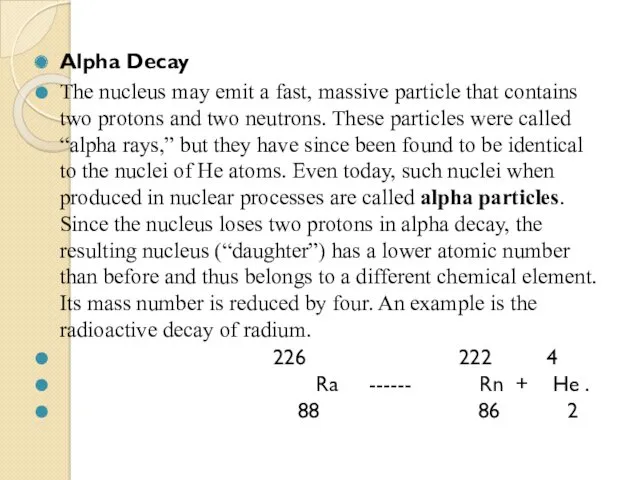
- 62. Alpha Decay The nucleus may emit a fast, massive particle that contains two protons and two
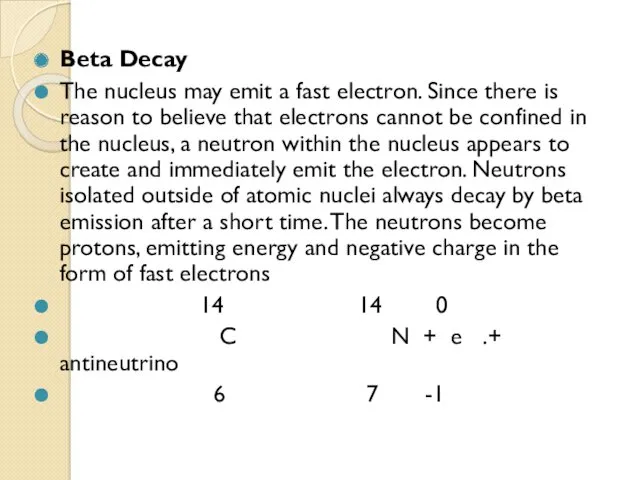
- 63. Beta Decay The nucleus may emit a fast electron. Since there is reason to believe that
- 65. Скачать презентацию




















 Механические колебания
Механические колебания Архимедова сила. Подготовка к ГИА
Архимедова сила. Подготовка к ГИА Бытовая швейная машина. Правила техники безопасности при работе на швейной машине. 5 класс
Бытовая швейная машина. Правила техники безопасности при работе на швейной машине. 5 класс Звуковые волны. Ультразвук
Звуковые волны. Ультразвук разработка к уроку на тему Электролизация тел 8 класс
разработка к уроку на тему Электролизация тел 8 класс Система EDS (Elektronische Differentialsperre) для автомобилей
Система EDS (Elektronische Differentialsperre) для автомобилей Преломление света. Закон преломления света. 9 класс
Преломление света. Закон преломления света. 9 класс Определение твёрдости металлов и сплавов
Определение твёрдости металлов и сплавов Высота всасывания насоса. Условия бескавитационной работы насоса. (Лекция 8)
Высота всасывания насоса. Условия бескавитационной работы насоса. (Лекция 8) Моторамы. Назначение моторам
Моторамы. Назначение моторам Архимедова сила
Архимедова сила Теплоизоляция домов
Теплоизоляция домов Отчет по производственной практике по специализации: Слесарь по контрольно-измерительным приборам и автоматике
Отчет по производственной практике по специализации: Слесарь по контрольно-измерительным приборам и автоматике Формирование навыков смыслового чтения и работы с текстом на уроках физики (Часть2)
Формирование навыков смыслового чтения и работы с текстом на уроках физики (Часть2) Интерактивная мозаика-2017. Знатоки физики 8 класс
Интерактивная мозаика-2017. Знатоки физики 8 класс Работа электрического поля при перемещении заряда
Работа электрического поля при перемещении заряда Резьбовые соединения
Резьбовые соединения Ремонт авиационной техники
Ремонт авиационной техники Рентгеноструктурный анализ
Рентгеноструктурный анализ Три состояния вещества
Три состояния вещества Пасивні елементи засобів вимірювань
Пасивні елементи засобів вимірювань Винаходи під час промислового перевороту в Англії
Винаходи під час промислового перевороту в Англії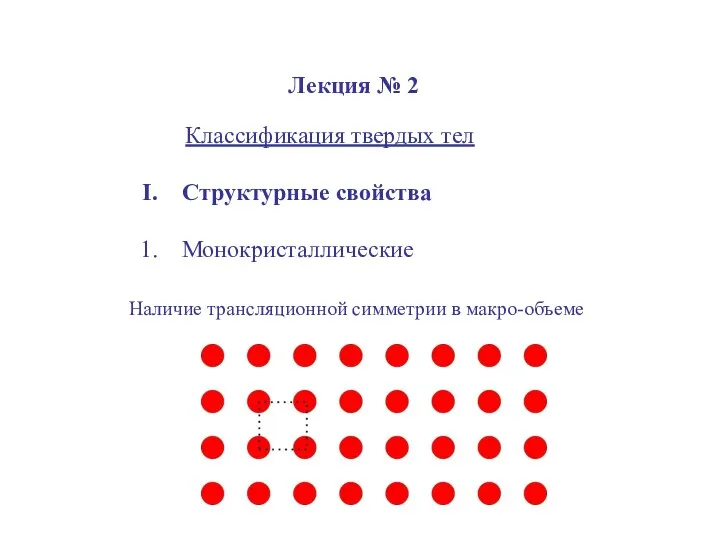 Классификация твердых тел
Классификация твердых тел Давление на дне морей и океанов
Давление на дне морей и океанов Динамика. Законы динамики
Динамика. Законы динамики Зубчатые передачи
Зубчатые передачи Буксовые узлы. Устройство. Наблюдение и уход за буксами в эксплуатации. Основные детали и их неисправности
Буксовые узлы. Устройство. Наблюдение и уход за буксами в эксплуатации. Основные детали и их неисправности Презентация к уроку Исследование капиллярных свойств столовых салфеток. Урок-контрольная закупка.
Презентация к уроку Исследование капиллярных свойств столовых салфеток. Урок-контрольная закупка.