Слайд 2

The Photoelectric Effect
In 1887 Hertz noticed, in the course of his
investigations, that a negatively charged electroscope could be discharged by shining ultraviolet light on it.
In 1899, Thomson showed that the emitted charges were electrons.
The emission of electrons from a substance due to light striking its surface came to be called the photoelectric effect.
The emitted electrons are often called photoelectrons to indicate their origin, but they are identical in every respect to all other electrons.
Слайд 3
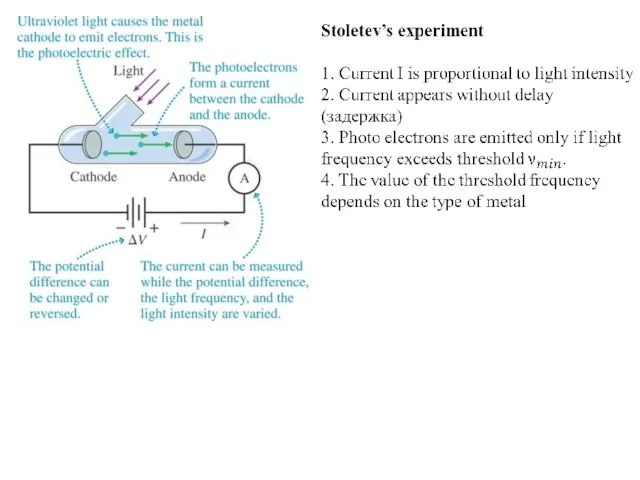
Слайд 4
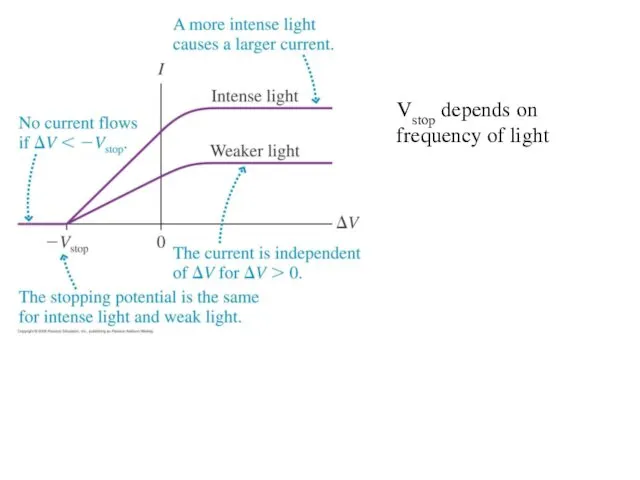
Vstop depends on frequency of light
Слайд 5
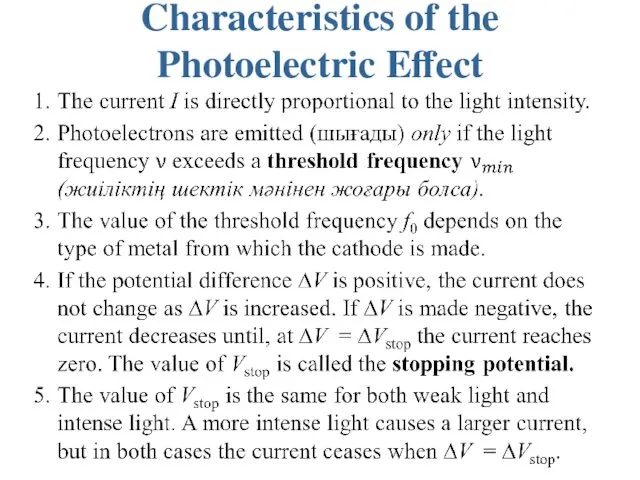
Characteristics of the Photoelectric Effect
Слайд 6
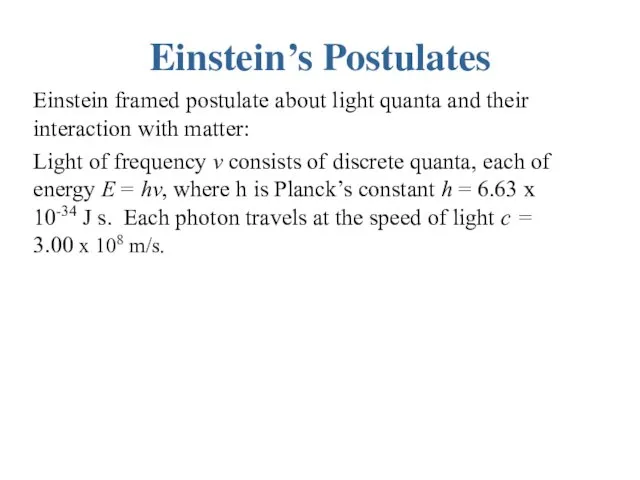
Einstein’s Postulates
Einstein framed postulate about light quanta and their interaction with
matter:
Light of frequency ν consists of discrete quanta, each of energy E = hν, where h is Planck’s constant h = 6.63 x 10-34 J s. Each photon travels at the speed of light c = 3.00 x 108 m/s.
Слайд 7
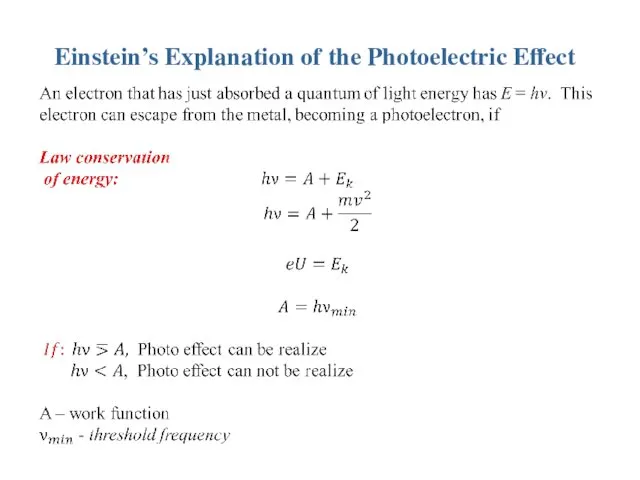
Einstein’s Explanation of the Photoelectric Effect
Слайд 8

Слайд 9








 Измерение децентрировки линз коллимационным и автоколлимационным методами
Измерение децентрировки линз коллимационным и автоколлимационным методами Радиолокация
Радиолокация Конструкция автомобиля. Полуоси
Конструкция автомобиля. Полуоси Іштен жанатын қозғалтқыштардың негізгі элементтері
Іштен жанатын қозғалтқыштардың негізгі элементтері Atomic theory and structure of an atom
Atomic theory and structure of an atom Сила давления жидкости на плоские и криволинейные стенки
Сила давления жидкости на плоские и криволинейные стенки Презентация урока по физике для 8 класса на тему Двигатель внутреннего сгорания
Презентация урока по физике для 8 класса на тему Двигатель внутреннего сгорания Градуировка спектроскопа
Градуировка спектроскопа Новые средства измерения температуры АО НПП Эталон
Новые средства измерения температуры АО НПП Эталон Распределители с закрытым центром. Описание функционирования
Распределители с закрытым центром. Описание функционирования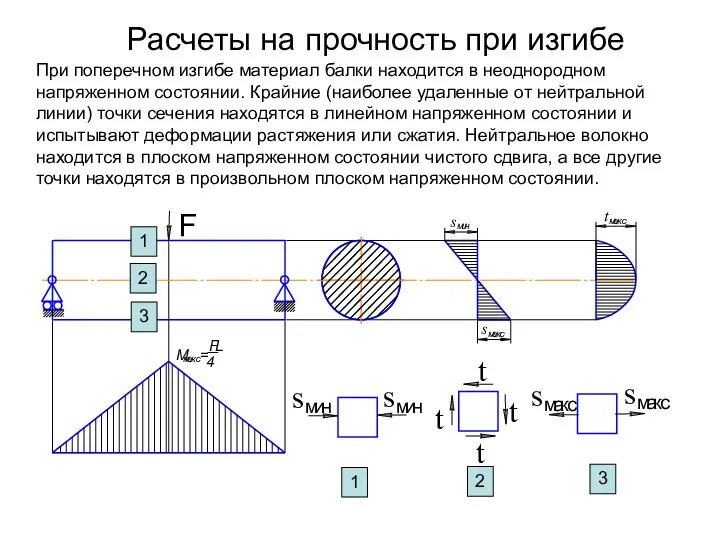 Расчеты на прочность при изгибе
Расчеты на прочность при изгибе Бойове застосування КЗА 86Ж6. Система електроживлення, вентиляції, кондиціювання. Технологічне обладнання 86Ж6. (Тема 9.4)
Бойове застосування КЗА 86Ж6. Система електроживлення, вентиляції, кондиціювання. Технологічне обладнання 86Ж6. (Тема 9.4) XWER381ADM0002070
XWER381ADM0002070 Теплотехника. Энтропия и первое начало термодинамики. (Лекция 4)
Теплотехника. Энтропия и первое начало термодинамики. (Лекция 4) Молекулярная физика
Молекулярная физика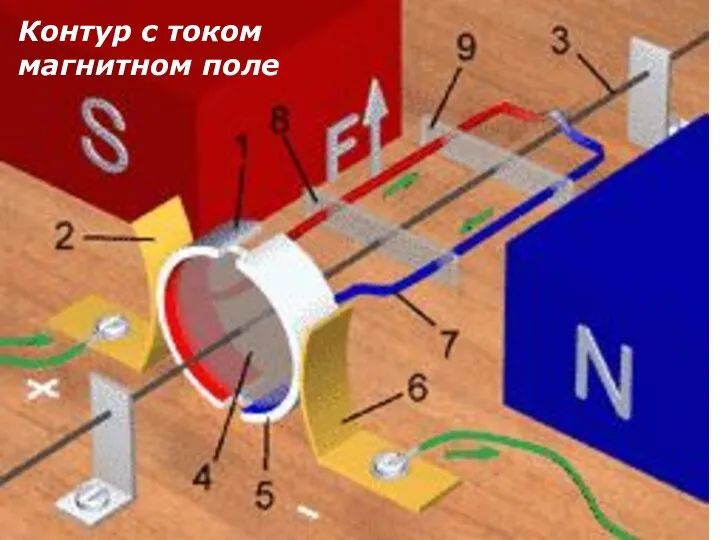 Контур с током в магнитном поле
Контур с током в магнитном поле Урок по физике 8 класс. Обобщение материала по теме: Изменения агрегатных состояний вещества
Урок по физике 8 класс. Обобщение материала по теме: Изменения агрегатных состояний вещества Радиоактивность
Радиоактивность Обучение физике в странах Европы
Обучение физике в странах Европы Организация технического обслуживания и ремонта автомобиля ЗИЛ 4331
Организация технического обслуживания и ремонта автомобиля ЗИЛ 4331 Квантовые системы. Распределение электронов в атоме. Квантовые числа. Принцип Паули. Спонтанное и вынужденное излучение. Лазеры
Квантовые системы. Распределение электронов в атоме. Квантовые числа. Принцип Паули. Спонтанное и вынужденное излучение. Лазеры Погрешности измерений при проведении школьного физического эксперимента
Погрешности измерений при проведении школьного физического эксперимента Высоковольтные выключатели
Высоковольтные выключатели Теория электрических цепей
Теория электрических цепей Тепловые двигатели
Тепловые двигатели Электроемкость. Конденсаторы. Энергия заряженного конденсатора
Электроемкость. Конденсаторы. Энергия заряженного конденсатора Гравиметрия, или гравиразведка. (Лекция 5)
Гравиметрия, или гравиразведка. (Лекция 5) Свет и цвет (презентация)
Свет и цвет (презентация)