Содержание
- 2. Plan Basic terms and concepts. The first law of thermodynamics. Enthalpy. Thermochemical equations. Thermochemistry. Caloric content
- 3. Basic terms and concepts
- 4. THE SUBJECT OF THERMODYNAMICS Energy is the capacity of a physical system to perform work. Energy
- 5. THE SUBJECT OF THERMODYNAMICS Thermal energy - form of energy associated with the motion of atoms,
- 6. THE SUBJECT OF THERMODYNAMICS Mechanical energy can be converted into thermal energy and back. The conversion
- 7. Work is done when a force applied to some object moves the object. For example, lifting
- 8. Heat (Q) describes energy in transit from a warmer body to a cooler body. The inernal
- 9. Generally in chemistry is not required to know the absolute value of internal energy . Most
- 10. Thermodynamics Thermodynamics is the branch of physical science that studies all forms of energy and their
- 11. Thermodynamics allows you to: 1) calculate the thermal effects of different processes; 2) predict whether the
- 12. Terms and concepts System - a collection of physical objects , separated from the environment. Environment
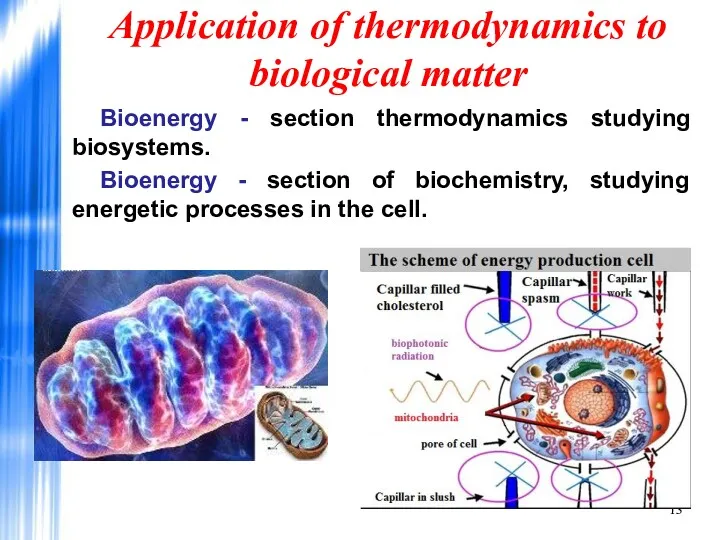
- 13. Application of thermodynamics to biological matter Bioenergy - section thermodynamics studying biosystems. Bioenergy - section of
- 14. Thermochemistry Thermochemistry - is a branch of chemistry that studies the effects of thermal and chemical
- 15. Thermodynamic parameters: extensive and intensive. If the system changes its parameters, then it takes a thermodynamic
- 16. Types of processes Isotermal process is a process in which temperature remains constant. Isobaric process is
- 17. Reversible process is a process that can be reversed by means of infinitesimal changes in some
- 18. Zero law of thermodynamics If each of the two thermodynamic system is in thermal equilibrium with
- 19. 1st law of thermodynamics 1st law of thermodynamics - is the law of conservation of energy.
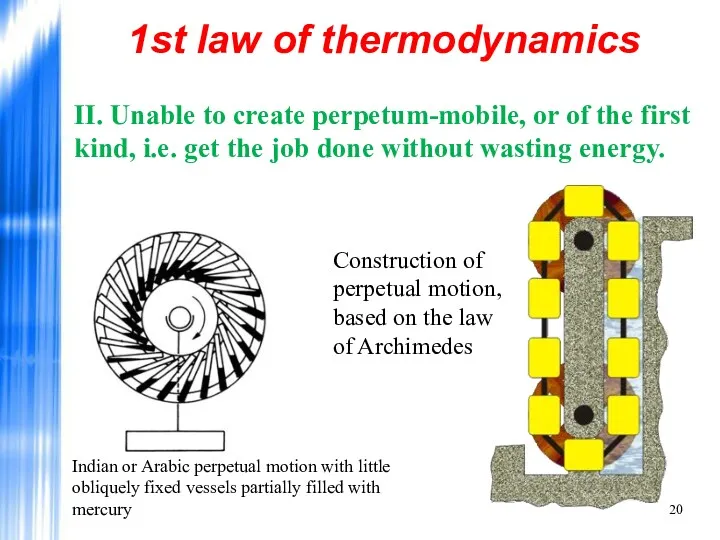
- 20. 1st law of thermodynamics II. Unable to create perpetum-mobile, or of the first kind, i.e. get
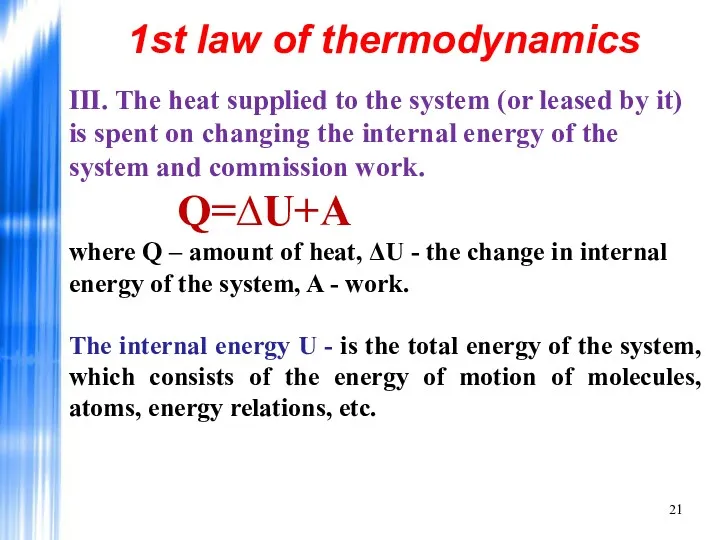
- 21. III. The heat supplied to the system (or leased by it) is spent on changing the
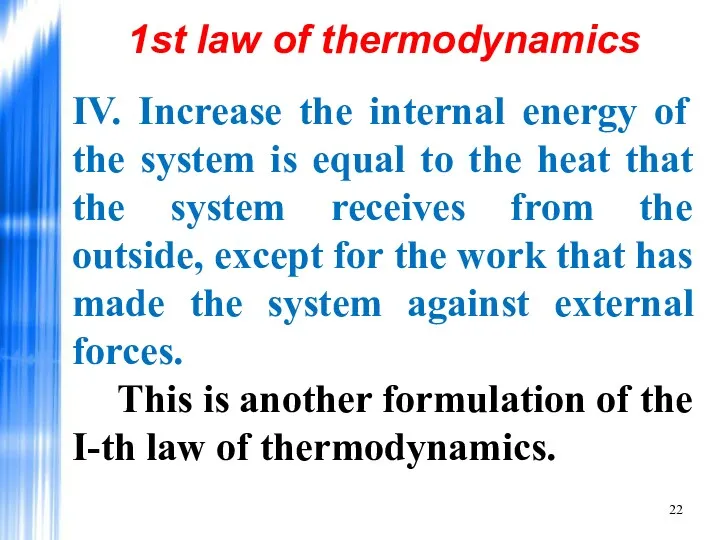
- 22. IV. Increase the internal energy of the system is equal to the heat that the system
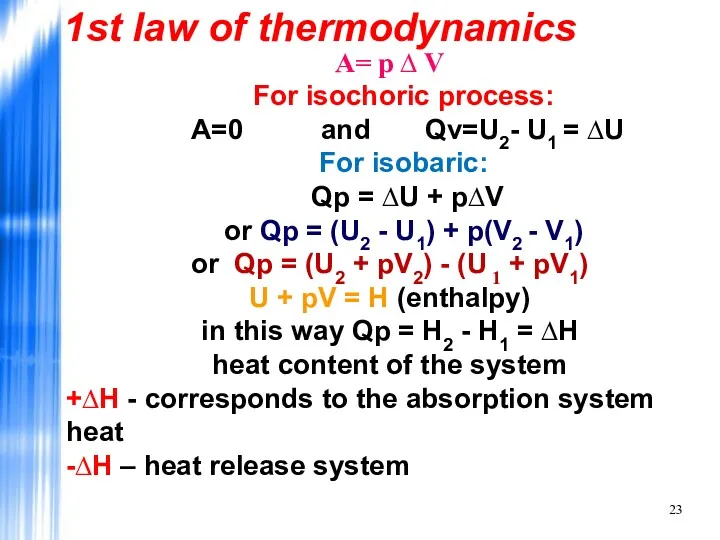
- 23. А= р ∆ V For isochoric process: A=0 and Qv=U2- U1 = ∆U For isobaric: Qp
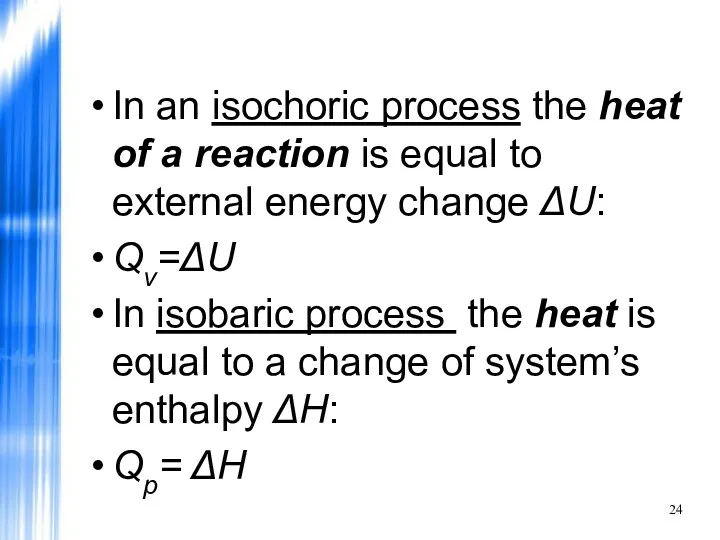
- 24. In an isochoric process the heat of a reaction is equal to external energy change ΔU:
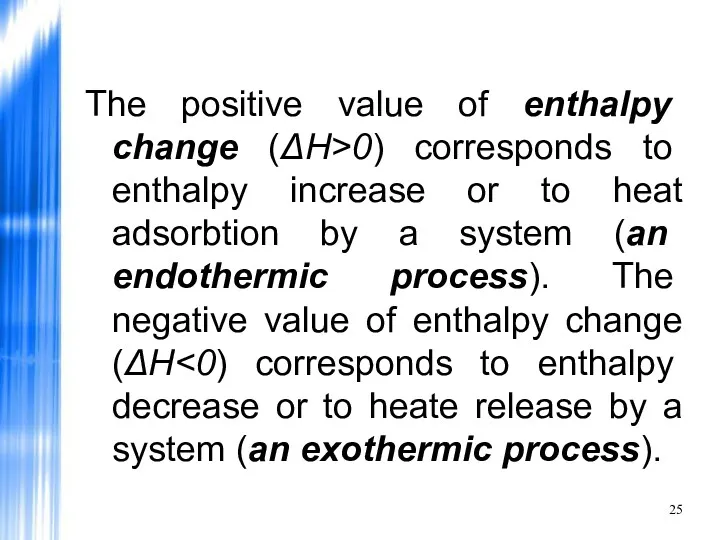
- 25. The positive value of enthalpy change (ΔH>0) corresponds to enthalpy increase or to heat adsorbtion by
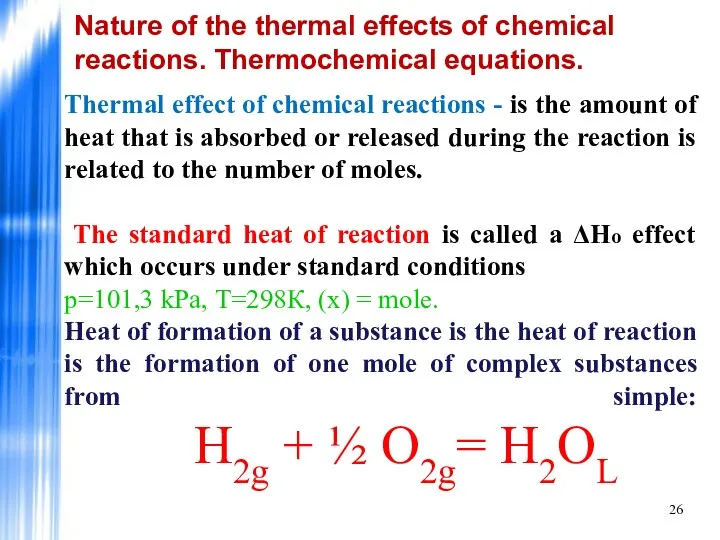
- 26. Nature of the thermal effects of chemical reactions. Thermochemical equations. Thermal effect of chemical reactions -
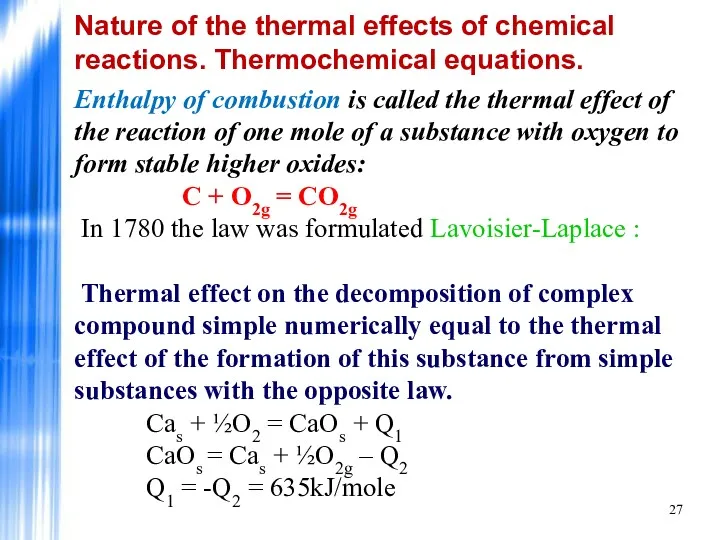
- 27. Enthalpy of combustion is called the thermal effect of the reaction of one mole of a
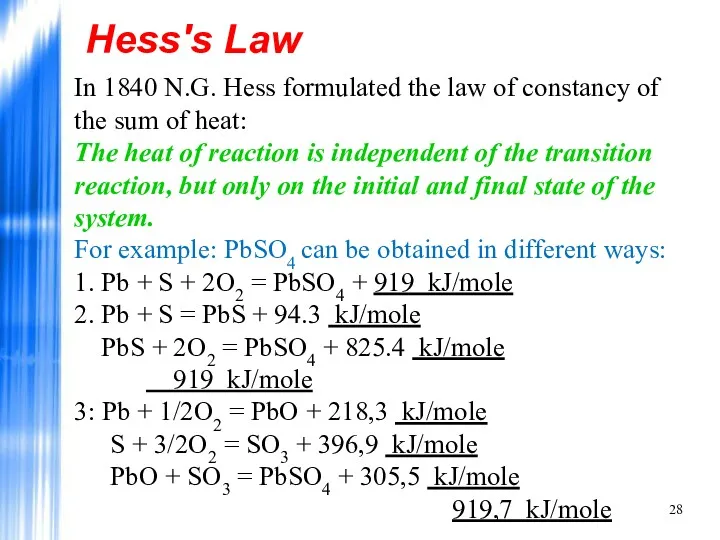
- 28. Hess's Law In 1840 N.G. Hess formulated the law of constancy of the sum of heat:
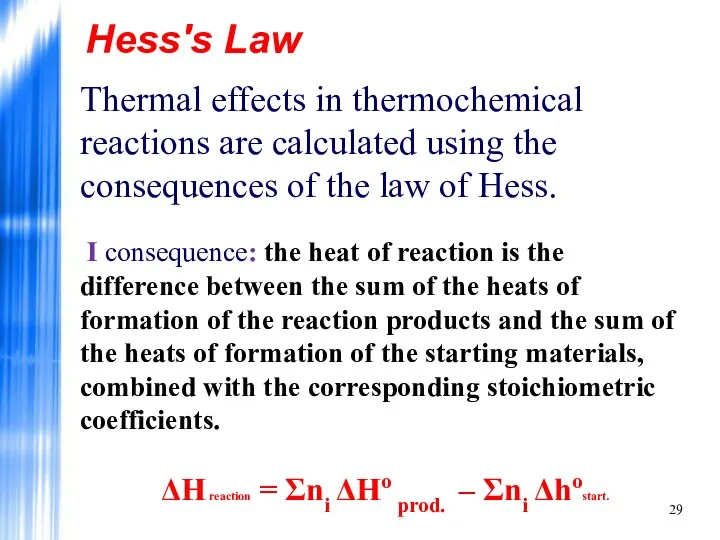
- 29. Hess's Law Thermal effects in thermochemical reactions are calculated using the consequences of the law of
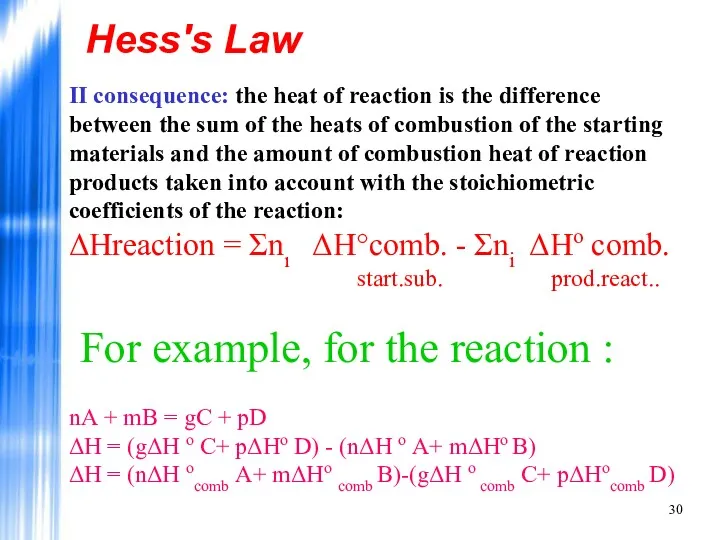
- 30. Hess's Law II consequence: the heat of reaction is the difference between the sum of the
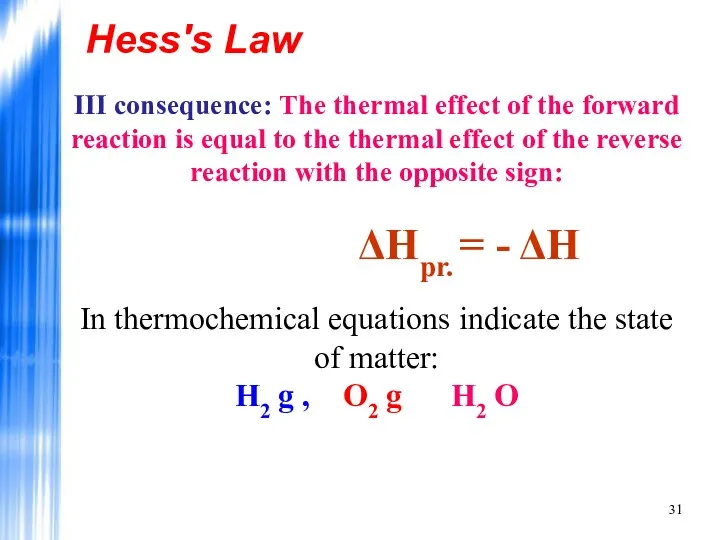
- 31. Hess's Law III consequence: The thermal effect of the forward reaction is equal to the thermal
- 32. Research of thermochemical calculations for the energy performance of biochemical processes Attached to the living organism
- 33. The human requirement for energy during the 24 h At easy work at sitting state (office
- 34. The energy is given mainly fats, proteins, carbohydrates: 39 kJ / g, 18 kJ / g,
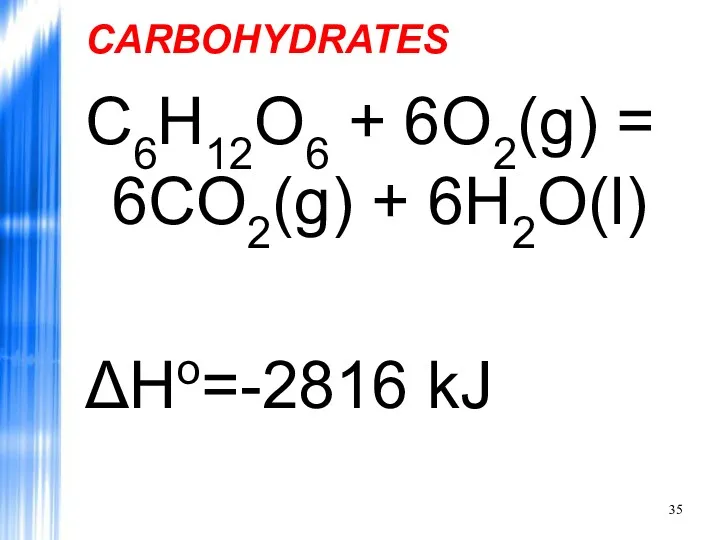
- 35. CARBOHYDRATES C6H12O6 + 6O2(g) = 6CO2(g) + 6H2O(l) ΔHo=-2816 kJ
- 36. FATS 2C57H110O6(s) + 163O2 → 114CO2+110H2O (l) ΔHo=-75520 kJ.
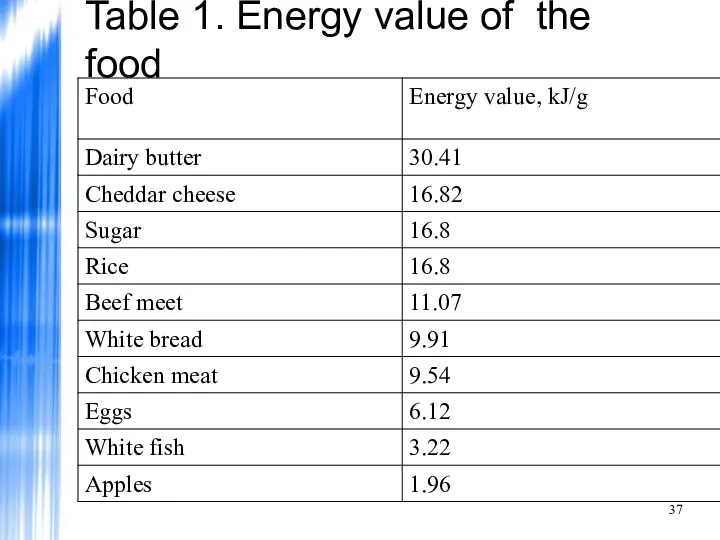
- 37. Table 1. Energy value of the food
- 38. 2nd law of thermodynamics heat can not of itself pass from cold to hot heat, leaving
- 39. Entropy Entropy is the property of a system which measures the degree of disorder or randomness
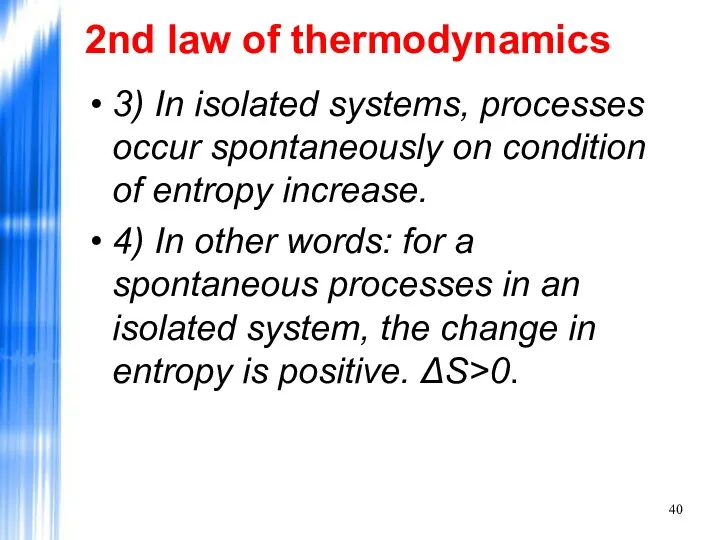
- 40. 2nd law of thermodynamics 3) In isolated systems, processes occur spontaneously on condition of entropy increase.
- 41. 2nd law of thermodynamics All real spontaneous processes - irreversible. Invertible only ideal process. In real
- 42. ΔS= S2-S1
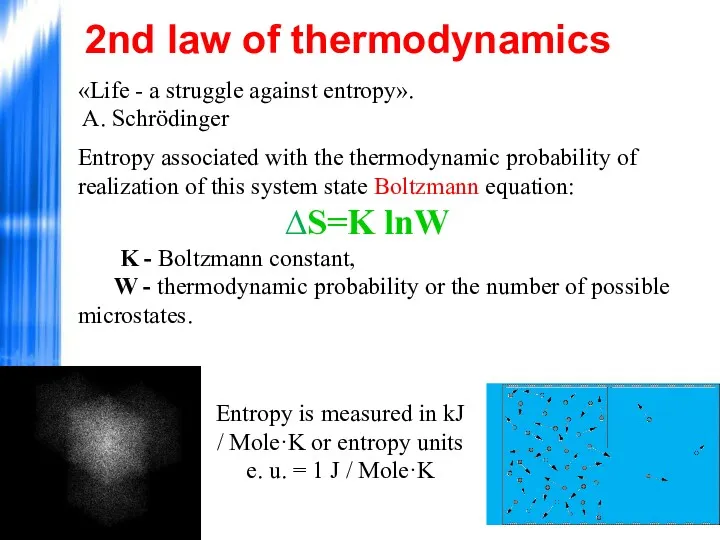
- 43. «Life - a struggle against entropy». A. Schrödinger Entropy associated with the thermodynamic probability of realization
- 44. 2nd law of thermodynamics The more disordered system the greater its entropy. Spontaneously reaching processes occur
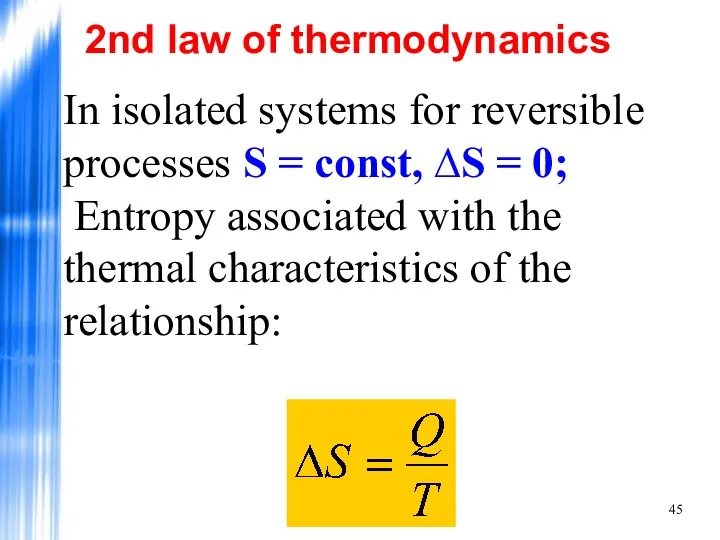
- 45. In isolated systems for reversible processes S = const, ∆S = 0; Entropy associated with the
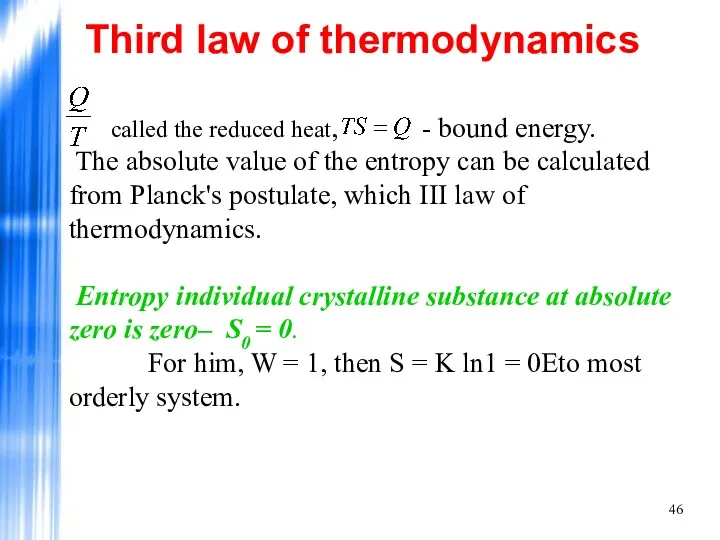
- 46. called the reduced heat, - bound energy. The absolute value of the entropy can be calculated
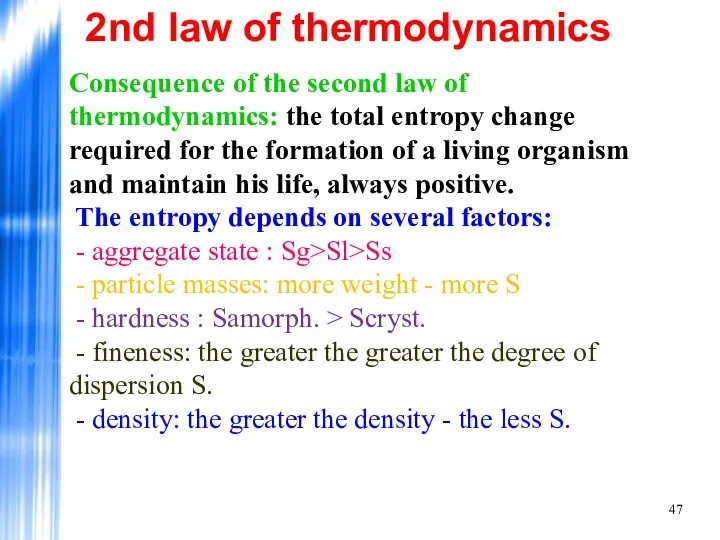
- 47. 2nd law of thermodynamics Consequence of the second law of thermodynamics: the total entropy change required
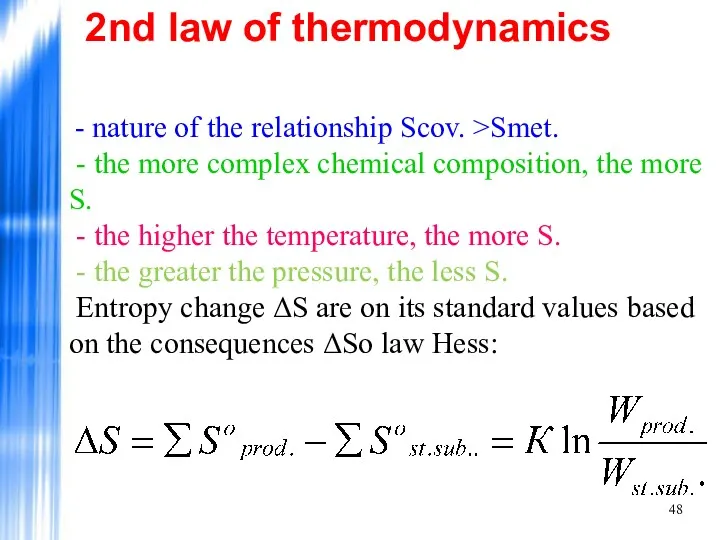
- 48. 2nd law of thermodynamics - nature of the relationship Scov. >Smet. - the more complex chemical
- 49. Free energy of system and free energy changes.The Gibbs’s equation
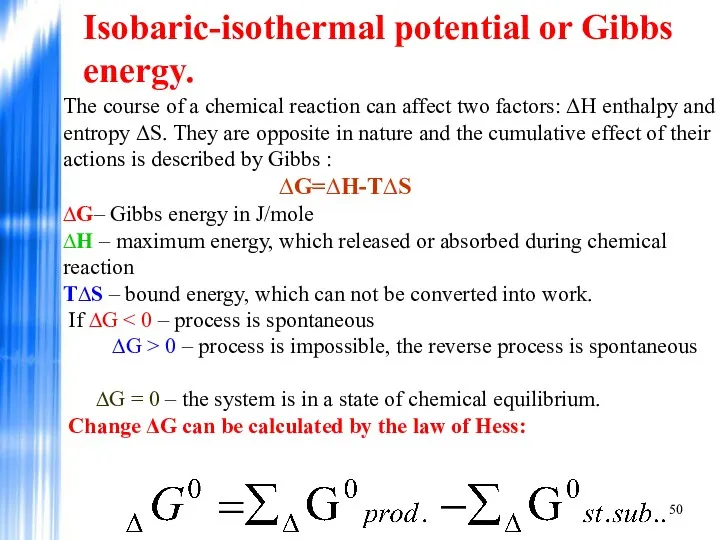
- 50. Isobaric-isothermal potential or Gibbs energy. The course of a chemical reaction can affect two factors: ΔH
- 51. ΔG ΔG>0 the process is impossible, the reverse process occurs spontaneously; ΔG=0 the system is an
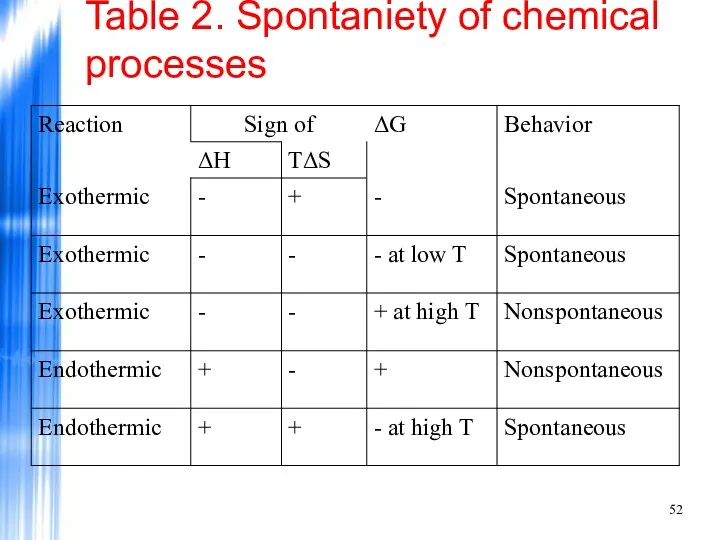
- 52. Table 2. Spontaniety of chemical processes
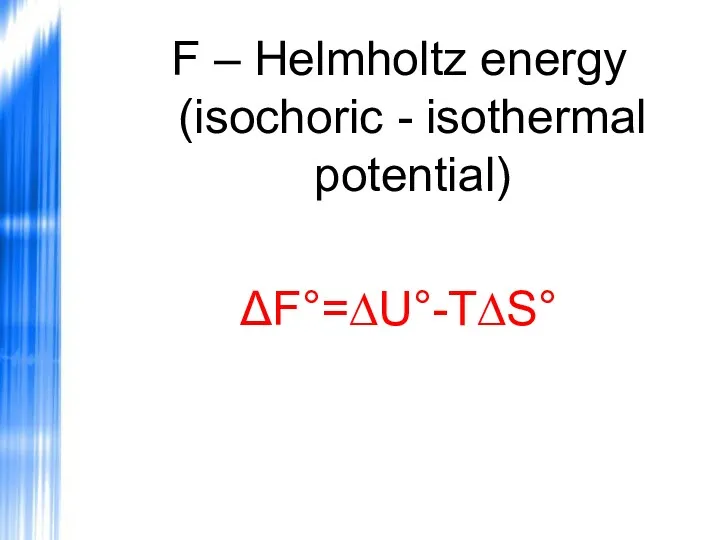
- 53. F – Helmholtz energy (isochoric - isothermal potential) ΔF°=∆U°-T∆S°
- 55. Скачать презентацию









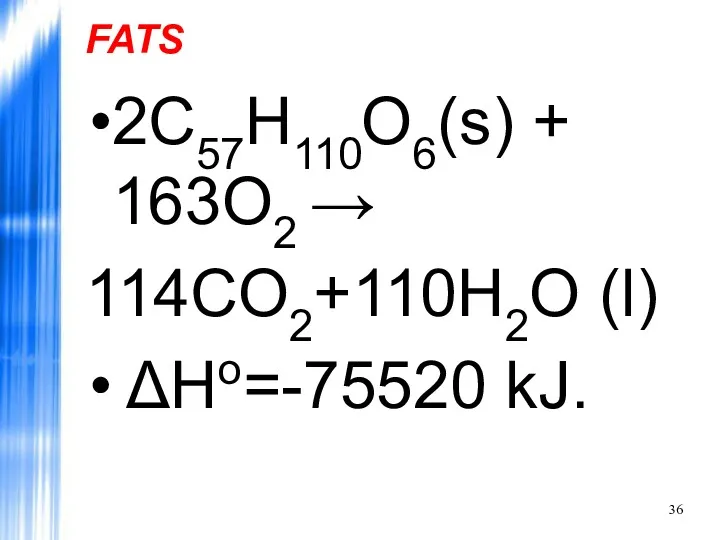


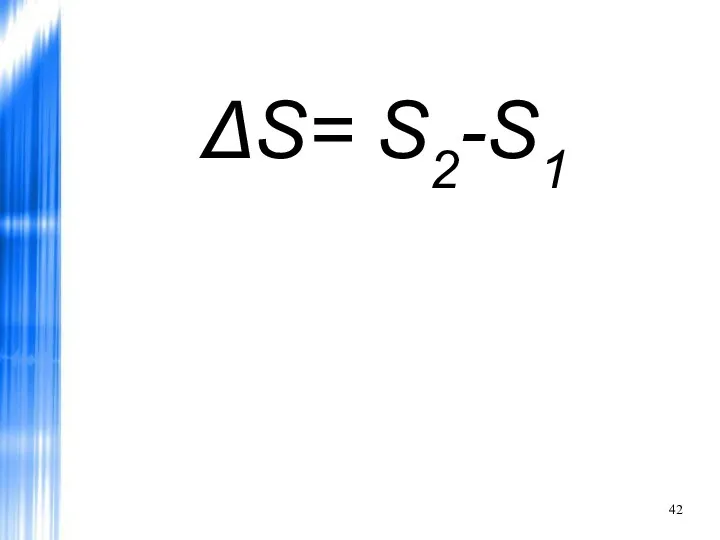


 Презентация по Физики 7 класс На тему Механическая работа и мощность.
Презентация по Физики 7 класс На тему Механическая работа и мощность. презентация к уроку плотность вещества
презентация к уроку плотность вещества Построение и применение комплексов радиорелейной, тропосферной, спутниковой связи
Построение и применение комплексов радиорелейной, тропосферной, спутниковой связи Презентация к уроку Виды сил. Равнодействующая сила. Правила сложения сил для 7 класса
Презентация к уроку Виды сил. Равнодействующая сила. Правила сложения сил для 7 класса Оборудование для смазочно-заправочных работ. Техническое обслуживание и ремонт автомобилей
Оборудование для смазочно-заправочных работ. Техническое обслуживание и ремонт автомобилей Устройство и назначение карданной передачи в автомобиле
Устройство и назначение карданной передачи в автомобиле Факторы влияющие на форму частотного отклика излучения лазера: Нелинейное усиление
Факторы влияющие на форму частотного отклика излучения лазера: Нелинейное усиление Температура. Связь температуры со скоростью теплового движения частиц
Температура. Связь температуры со скоростью теплового движения частиц Формула Максвелла для относительных скоростей
Формула Максвелла для относительных скоростей Електричний струм у газах
Електричний струм у газах Основы расчетов движения автомобилей по дорогам
Основы расчетов движения автомобилей по дорогам Энергия. Виды энергии. Презентация
Энергия. Виды энергии. Презентация Methods and technical means for using the energy of waves
Methods and technical means for using the energy of waves Излучение и спектры
Излучение и спектры Рабочие процессы дизельного двигателя
Рабочие процессы дизельного двигателя Устройство и назначение автосцепки вагонов
Устройство и назначение автосцепки вагонов Управляемость и проходимость автомобиля. Характеристика и показатели траекторной управляемости
Управляемость и проходимость автомобиля. Характеристика и показатели траекторной управляемости Теплообменные аппараты
Теплообменные аппараты Техническая информация по коммерческим автомобилям NRW 2019. Двигатели
Техническая информация по коммерческим автомобилям NRW 2019. Двигатели Электрическое поле в вакууме. (Тема 13)
Электрическое поле в вакууме. (Тема 13) Механические характеристики производственных механизмов и электродвигателей
Механические характеристики производственных механизмов и электродвигателей Гипоидная передача
Гипоидная передача Курс Атомные реакторы и ядерная энергетика. Лекция 3. Ядерная энергетика. Настоящее и будущее
Курс Атомные реакторы и ядерная энергетика. Лекция 3. Ядерная энергетика. Настоящее и будущее Дифракционная решетка
Дифракционная решетка Опиливание металла напильником. Классификация напильников
Опиливание металла напильником. Классификация напильников Резонанс, его польза и вред
Резонанс, его польза и вред Поршни. Основные части днища
Поршни. Основные части днища Работа электрического поля. 10 класс
Работа электрического поля. 10 класс