Critical Path Research: Getting New Technology from Bench to Bedside A Device. Perspective FDA Science Board November 5, 2004 презентация
Содержание
- 2. Role of FDA Establish reasonable assurance of the safety and effectiveness of medical devices marketed in
- 3. What is a “Device”?
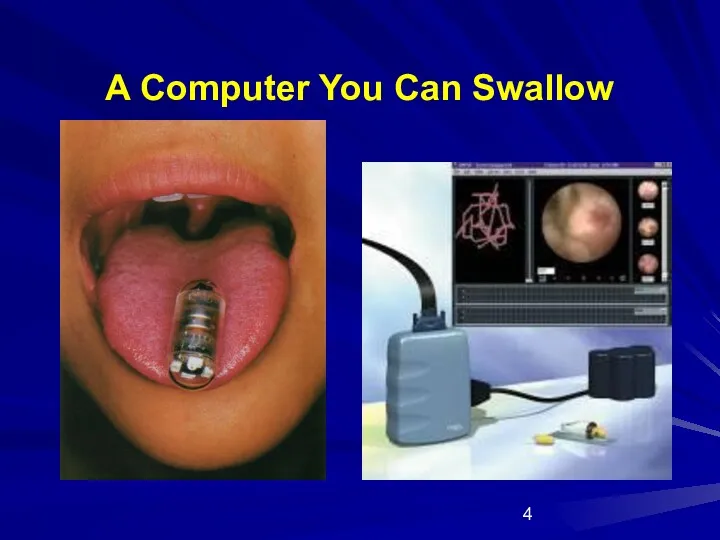
- 4. A Computer You Can Swallow
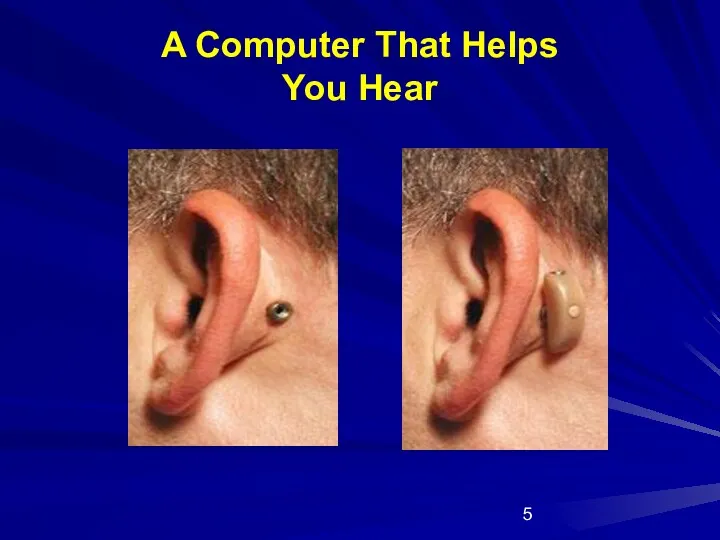
- 5. A Computer That Helps You Hear
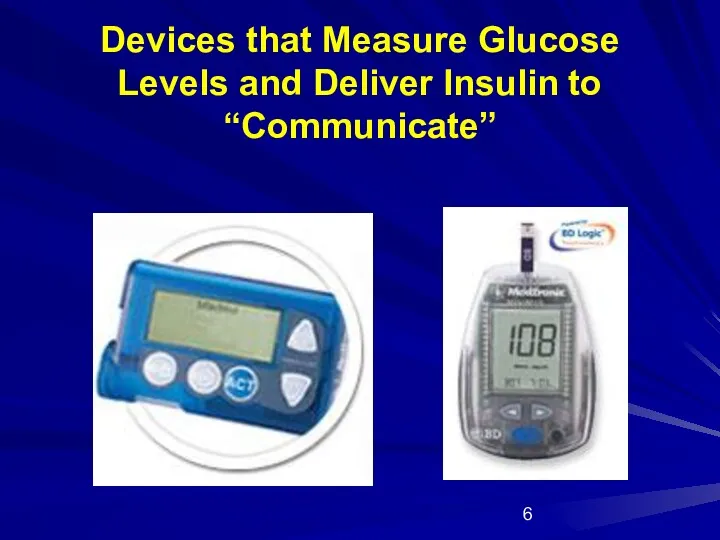
- 6. Devices that Measure Glucose Levels and Deliver Insulin to “Communicate”
- 7. Miniaturized Electrical Stimulators Pacemakers
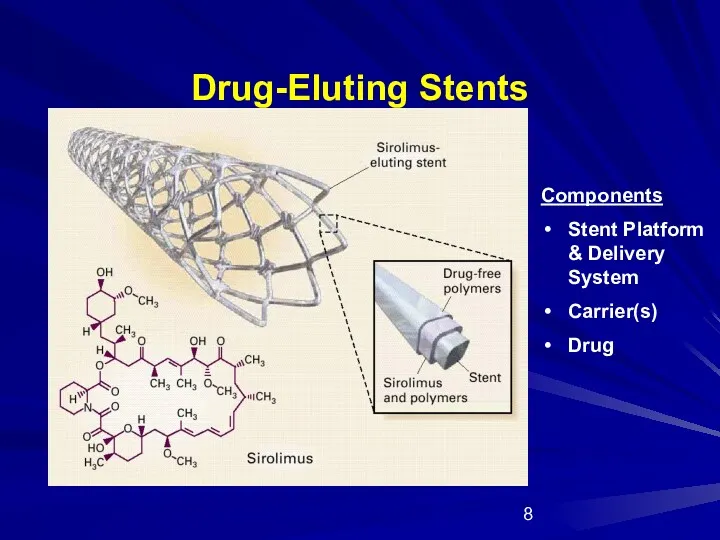
- 8. Drug-Eluting Stents Components Stent Platform & Delivery System Carrier(s) Drug
- 9. New Technology Important Trends Miniaturization Intelligent Devices Designed for Consumer Use Minimally invasive Biotechnology Revolution Genomics,
- 10. CDRH Vision – Total Product Life Cycle
- 11. Devices are Different Drugs Pure molecules Toxicology Short half-life Long market life Drug interactions Wrong Drug
- 12. Critical Path is Different for Devices Device Regulation Least Burdensome Provision of FDAMA Quality Systems and
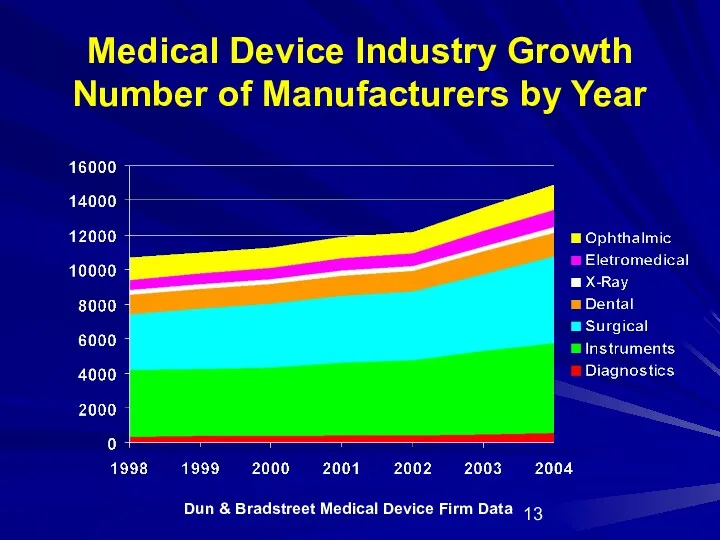
- 13. Dun & Bradstreet Medical Device Firm Data Medical Device Industry Growth Number of Manufacturers by Year
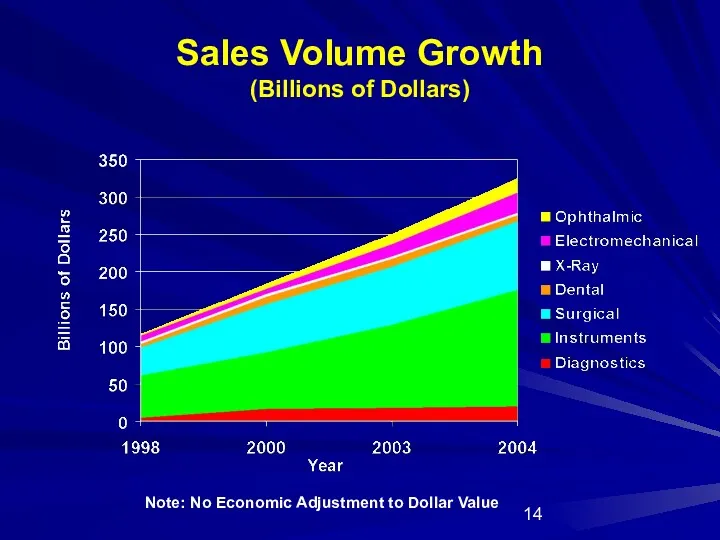
- 14. Sales Volume Growth (Billions of Dollars) Note: No Economic Adjustment to Dollar Value
- 15. Device Industry Continues to Grow in FY 04 Dun and Bradstreet FY 04 data shows the
- 16. Innovative Science-based Strategies at Work Leveraging Breast Cancer (DMIST): Screening and Digital Mammography Medical Device Fellowship
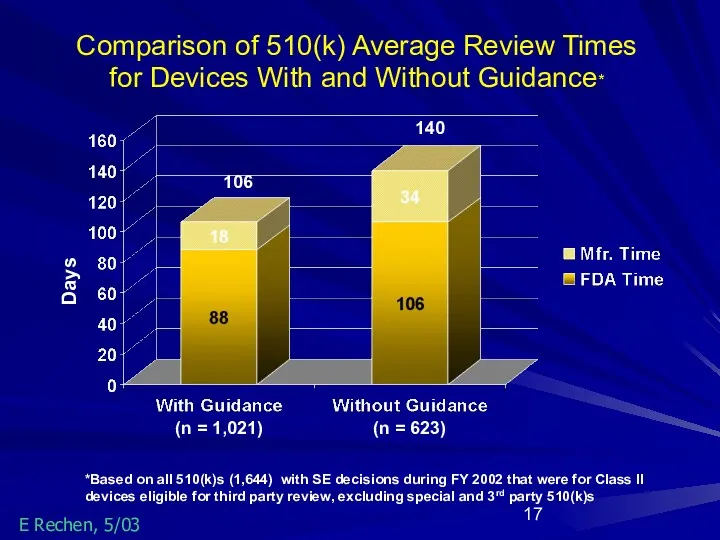
- 17. Days *Based on all 510(k)s (1,644) with SE decisions during FY 2002 that were for Class
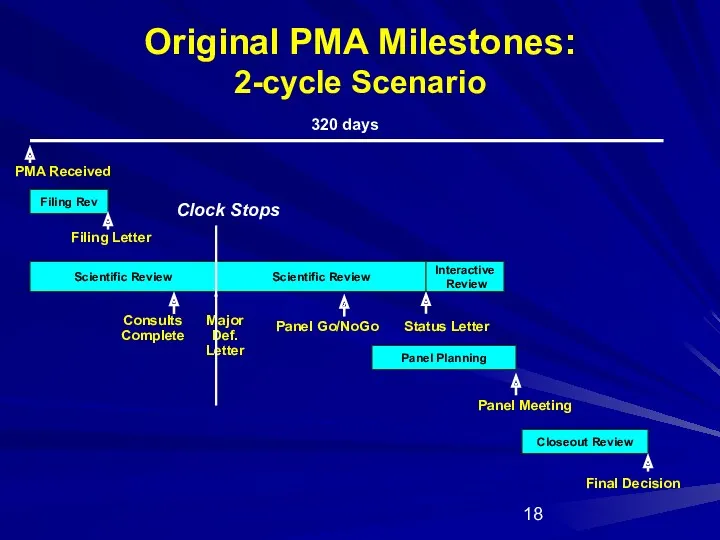
- 18. Original PMA Milestones: 2-cycle Scenario Filing Rev Scientific Review Panel Planning Closeout Review PMA Received Panel
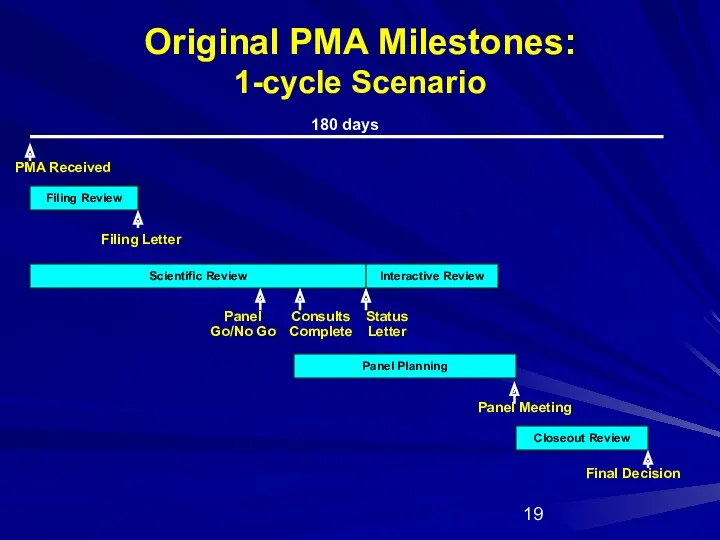
- 19. Original PMA Milestones: 1-cycle Scenario Filing Review Scientific Review Panel Planning Closeout Review PMA Received Panel
- 20. The rest of the story…
- 21. Drug-coated stents may face additional FDA scrutiny FDA Advises Physicians of Adverse Events Associated with Cordis
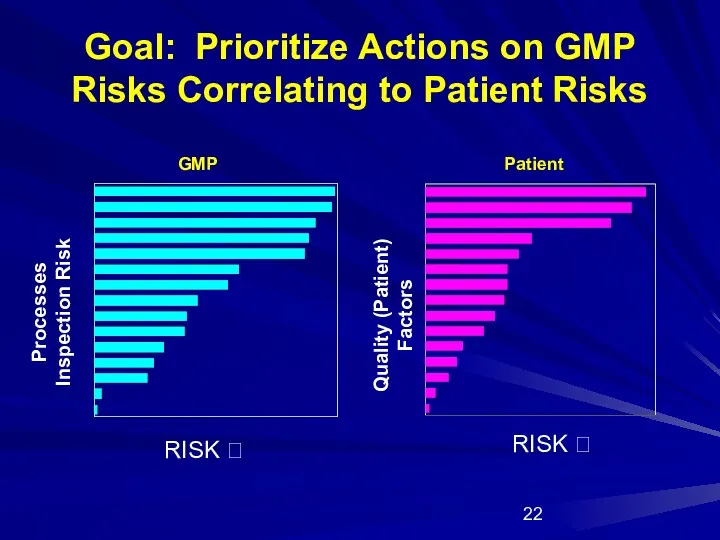
- 22. Goal: Prioritize Actions on GMP Risks Correlating to Patient Risks RISK ? Processes Inspection Risk RISK
- 23. Postmarket Questions of Interest Long Term Safety Performance in Community Practice Change in User Setting Rare/Unexpected
- 24. Achieving Pre/Postmarket Balance
- 25. Why Balance Works Speeds Product to Market by Moving Some Premarket Requirements to Postmarket Offers Added
- 26. Postmarket Studies - Present Ill-Conceived Not Initiated Not Completed Not Tracked Not Enforced
- 27. Postmarket Studies - Future Better Designs Standardized Reporting System Better Tracking Make Status of Studies Public
- 28. Life Sciences Laboratory Awards 2004, GSA Construction Excellence, Projects Over $25 Million 2004, Washington Building Council,
- 29. Critical Path Projects Being Developed Establishing a pedigreed and credentialed blood panel that could be used
- 30. Critical Path Projects Being Developed Establishing agreed pathways for the statistical validation of surrogate markers Working
- 31. Summary Steady progress towards meeting review performance goals and TPLC strategic goals Success is achievable but
- 33. Скачать презентацию



















 Сущность, средства и способы управления эксплуатацией космических средств. Лекция №04
Сущность, средства и способы управления эксплуатацией космических средств. Лекция №04 Многопользовательский доступ к базам данных
Многопользовательский доступ к базам данных Медійні лайфхаки
Медійні лайфхаки Мобильная электронная подпись (МЭП). Инструмент подписания электронных документов
Мобильная электронная подпись (МЭП). Инструмент подписания электронных документов Курс HTML и CSS. 2 занятие
Курс HTML и CSS. 2 занятие Язык программирования
Язык программирования Електронна пошта
Електронна пошта Файлы и папки.
Файлы и папки. Списки – способ упорядочивания информации
Списки – способ упорядочивания информации Операционные системы. Файловые системы. Загрузчики. Виртуальные среды
Операционные системы. Файловые системы. Загрузчики. Виртуальные среды Валидация и верификация
Валидация и верификация Роль информационной деятельности в современном обществе
Роль информационной деятельности в современном обществе Системное программное обеспечение. Операционные системы персональных компьютеров. (Лекция 6.2)
Системное программное обеспечение. Операционные системы персональных компьютеров. (Лекция 6.2) Organizational communication. Netiquette
Organizational communication. Netiquette 3D моделювання макету замку
3D моделювання макету замку Представления основанных на классах
Представления основанных на классах Самоорганизующиеся карты. Практика
Самоорганизующиеся карты. Практика Ai2 APP Inventor. Переводчик
Ai2 APP Inventor. Переводчик Обработка данных
Обработка данных Масштаб изображения. Что такое пиксель?
Масштаб изображения. Что такое пиксель? Языки программирования. (Лекция 7.4)
Языки программирования. (Лекция 7.4) Ajax. Идея заложенная в Аjax
Ajax. Идея заложенная в Аjax ГИА по информатике: особенности контрольно-измерительных материалов ЕГЭ и ОГЭ в 2021 году
ГИА по информатике: особенности контрольно-измерительных материалов ЕГЭ и ОГЭ в 2021 году Программа CHEM3D
Программа CHEM3D Сетевые атаки
Сетевые атаки Системы управления базами данных. База данных в Access
Системы управления базами данных. База данных в Access Модель взаимодействия открытых систем
Модель взаимодействия открытых систем Сравнительный анализ дизайна интернет-сайтов
Сравнительный анализ дизайна интернет-сайтов