- Главная
- Математика
- Group theory. Symmetry in coordination chemistry

Содержание
- 3. Symmetry in Coordination Chemistry Symmetry in Coordination Chemistry provides a comprehensive discussion of molecular symmetry. It
- 4. Symmetry is important to chemistry because it undergirds essentially all specific interactions between molecules in nature
- 5. Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according
- 6. This group is called the point group of that molecule, because the set of symmetry operations
- 9. In mathematics and abstract algebra, group theory studies the algebraic structures known as groups. The concept
- 10. One of the most important mathematical achievements of the 20th century[1] was the collaborative effort, taking
- 12. Скачать презентацию
Symmetry in Coordination Chemistry
Symmetry in Coordination Chemistry provides a comprehensive discussion
Symmetry in Coordination Chemistry
Symmetry in Coordination Chemistry provides a comprehensive discussion
Symmetry is important to chemistry because it undergirds essentially all specific interactions between molecules in
Symmetry is important to chemistry because it undergirds essentially all specific interactions between molecules in
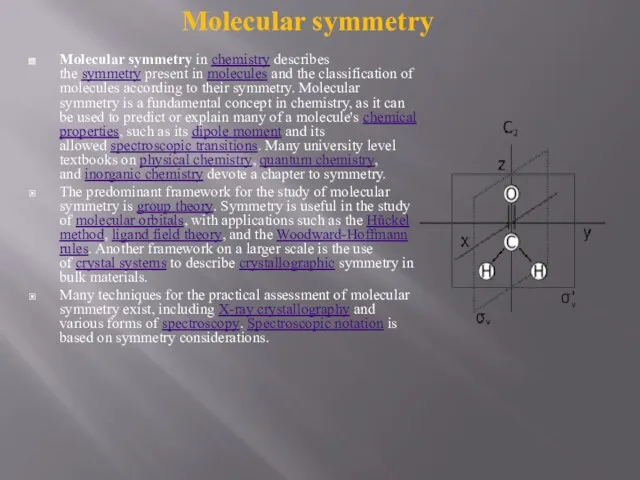
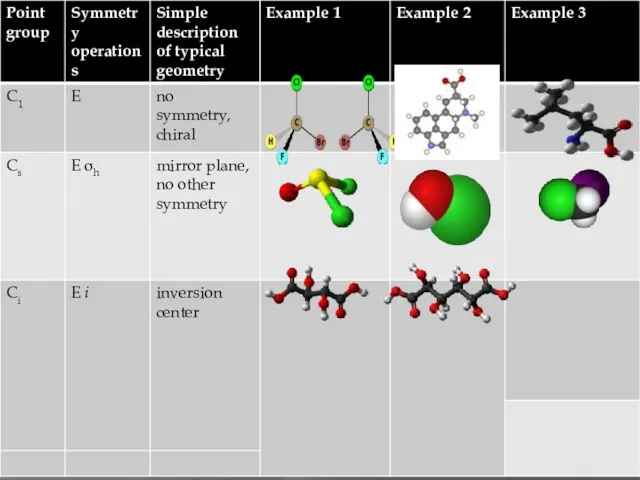
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their
The predominant framework for the study of molecular symmetry is group theory. Symmetry is useful in the study of molecular orbitals, with applications such as the Hückel method, ligand field theory, and the Woodward-Hoffmann rules. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materials.
Many techniques for the practical assessment of molecular symmetry exist, including X-ray crystallography and various forms of spectroscopy. Spectroscopic notation is based on symmetry considerations.
Molecular symmetry
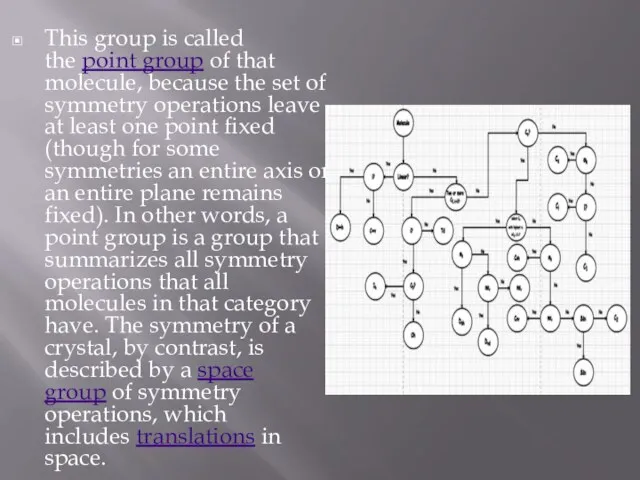
This group is called the point group of that molecule, because the set
This group is called the point group of that molecule, because the set
In mathematics and abstract algebra, group theory studies the algebraic structures known as groups. The concept of a group
In mathematics and abstract algebra, group theory studies the algebraic structures known as groups. The concept of a group
Various physical systems, such as crystals and the hydrogen atom, may be modelled by symmetry groups. Thus group theory and the closely related representation theory have many important applications in physics, chemistry, and materials science. Group theory is also central to public key cryptography.
This article covers advanced notions. For basic topics, see Group (mathematics).
For group theory in social scienspaces, can all be seen as groups endowed with additionalces, see social group.
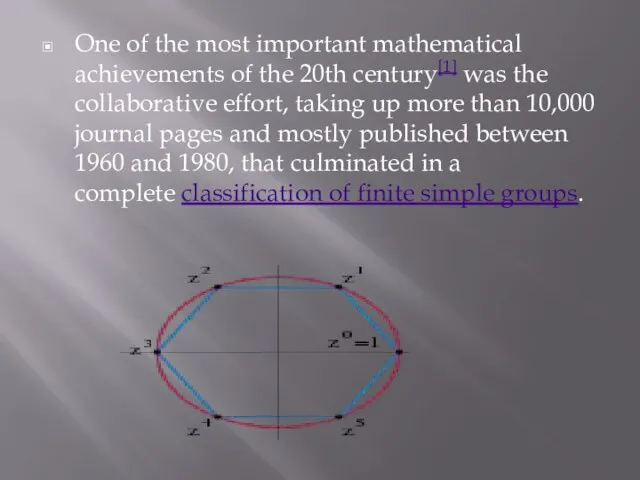
One of the most important mathematical achievements of the 20th century[1] was
One of the most important mathematical achievements of the 20th century[1] was





 Приемы умственной деятельности. Логика для младших школьников
Приемы умственной деятельности. Логика для младших школьников Нумерація чисел першої сотні. Нумераційна таблиця. Знаходження невідомого доданка. Урок №106
Нумерація чисел першої сотні. Нумераційна таблиця. Знаходження невідомого доданка. Урок №106 Трапеция
Трапеция Штей сызылған бұрыш туралы теорема
Штей сызылған бұрыш туралы теорема Презентация Устный счет до 5
Презентация Устный счет до 5 Теория статистических гипотез. Проверка статистических гипотез. Основы теории корреляции
Теория статистических гипотез. Проверка статистических гипотез. Основы теории корреляции Использование свойств функций при решении уравнений и неравенств
Использование свойств функций при решении уравнений и неравенств Показательные неравенства
Показательные неравенства Ломаные и многоугольники
Ломаные и многоугольники Метод координат
Метод координат Решение задач с помощью уравнений
Решение задач с помощью уравнений Умножение и деление десятичной дроби на разрядную единицу
Умножение и деление десятичной дроби на разрядную единицу Случаи вычитания 12 -
Случаи вычитания 12 - Десятичные дроби. 5 класс
Десятичные дроби. 5 класс Готовимся к ЕГЭ. Стереометрия
Готовимся к ЕГЭ. Стереометрия Треугольник. Правило треугольника
Треугольник. Правило треугольника Clipping summary
Clipping summary Решение задач на нахождение площади геометрических фигур на сетке. ОГЭ
Решение задач на нахождение площади геометрических фигур на сетке. ОГЭ Геометрия в древние и новые века. 7 класс
Геометрия в древние и новые века. 7 класс Координатная плоскость. 6 класс
Координатная плоскость. 6 класс Метрологические характеристики технических измерений
Метрологические характеристики технических измерений Десятая проблема Гильберта
Десятая проблема Гильберта Аналитическая геометрия
Аналитическая геометрия Эколого-математический брейн-ринг
Эколого-математический брейн-ринг Игра-тренажёр Весёлое путешествие Винни Пуха
Игра-тренажёр Весёлое путешествие Винни Пуха Методологические основы правовой статистики
Методологические основы правовой статистики Расшифровка ребусов
Расшифровка ребусов Сравнение функций
Сравнение функций