A randomized, double-blind, placebo-controlled trial of low-dose methotrexate for the prevention of atherosclerotic events презентация
Содержание
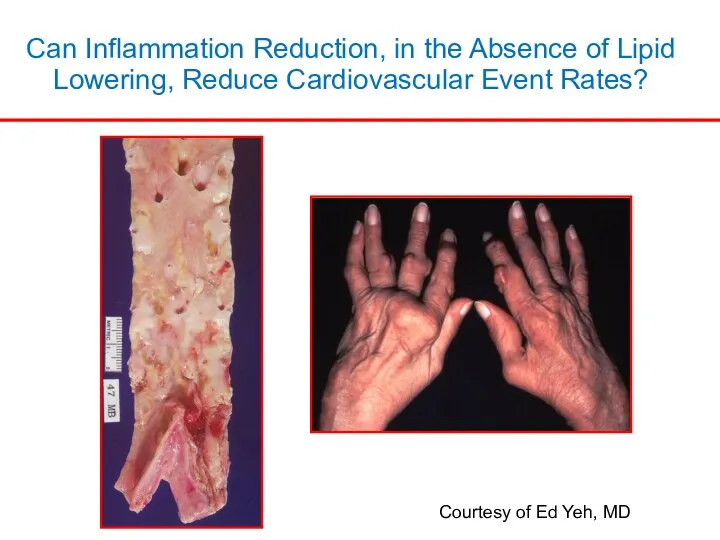
- 2. Can Inflammation Reduction, in the Absence of Lipid Lowering, Reduce Cardiovascular Event Rates? Courtesy of Ed
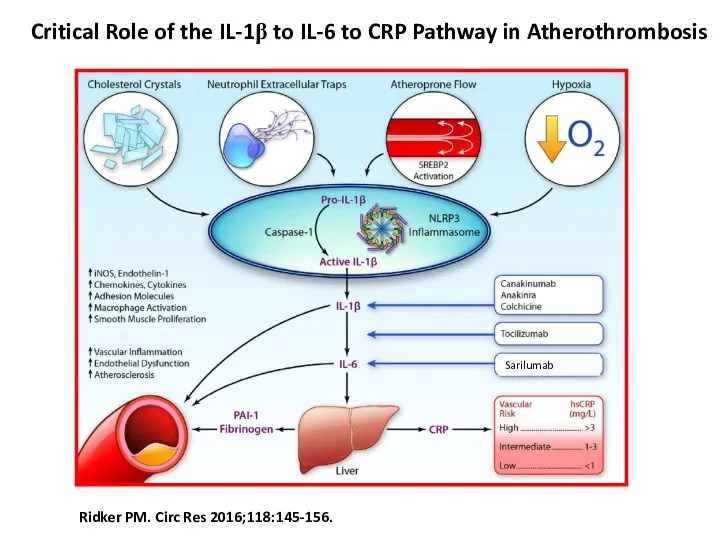
- 3. Ridker PM. Circ Res 2016;118:145-156. Critical Role of the IL-1β to IL-6 to CRP Pathway in
- 4. Interleukin-1β Inhibition IL-1β IL-6 hsCRP 15-17% reduction in MACE and MACE+ Low-Dose Methotrexate IL-1β IL-6 hsCRP
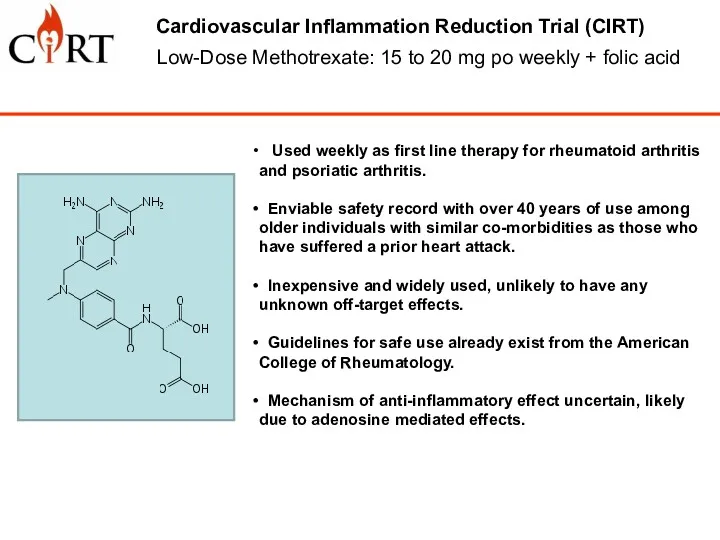
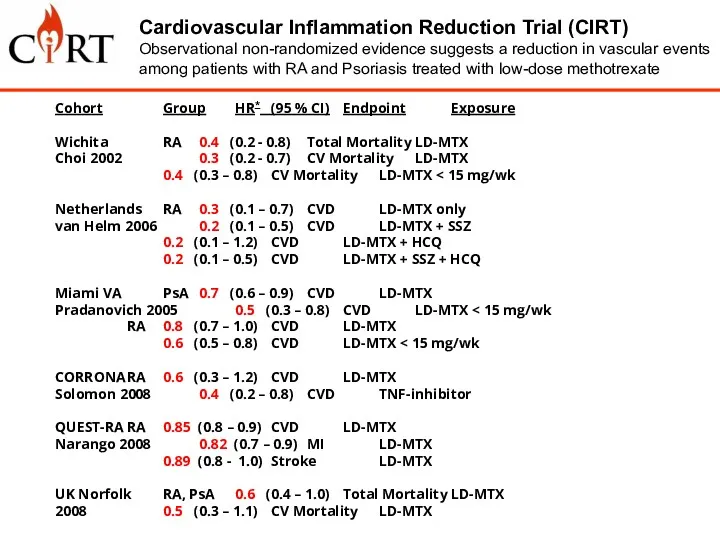
- 5. Low-Dose Methotrexate: 15 to 20 mg po weekly + folic acid Cardiovascular Inflammation Reduction Trial (CIRT)
- 6. Cohort Group HR* (95 % CI) Endpoint Exposure Wichita RA 0.4 (0.2 - 0.8) Total Mortality
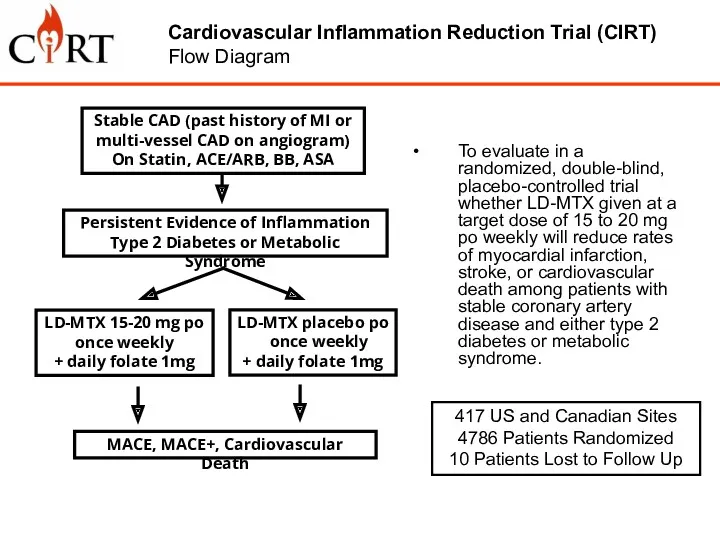
- 7. To evaluate in a randomized, double-blind, placebo-controlled trial whether LD-MTX given at a target dose of
- 8. aged 18 years and over have suffered a documented myocardial infarction or have multi-vessel CAD on
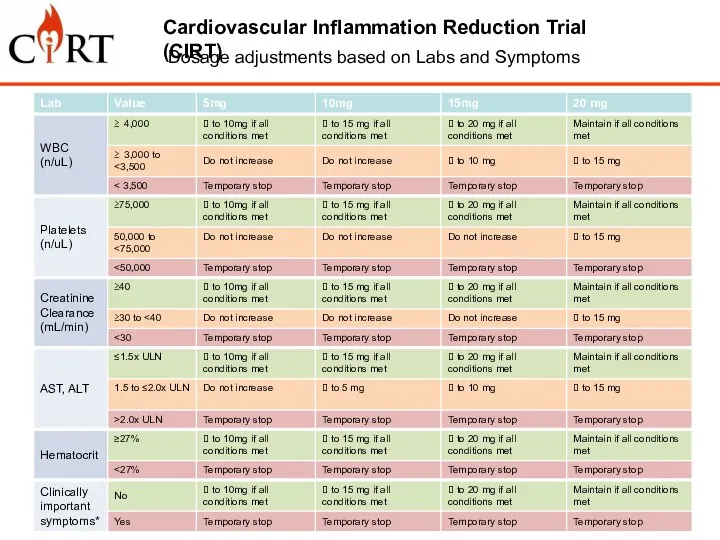
- 9. Cardiovascular Inflammation Reduction Trial (CIRT) Dosage adjustments based on Labs and Symptoms
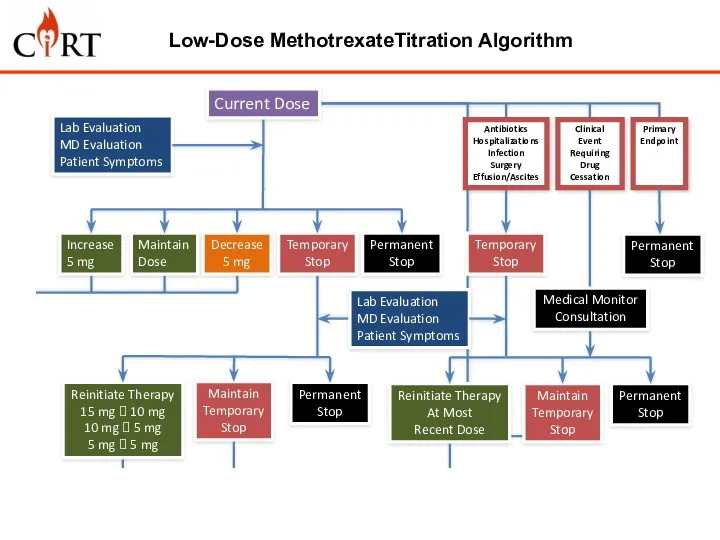
- 10. Low-Dose MethotrexateTitration Algorithm Medical Monitor Consultation
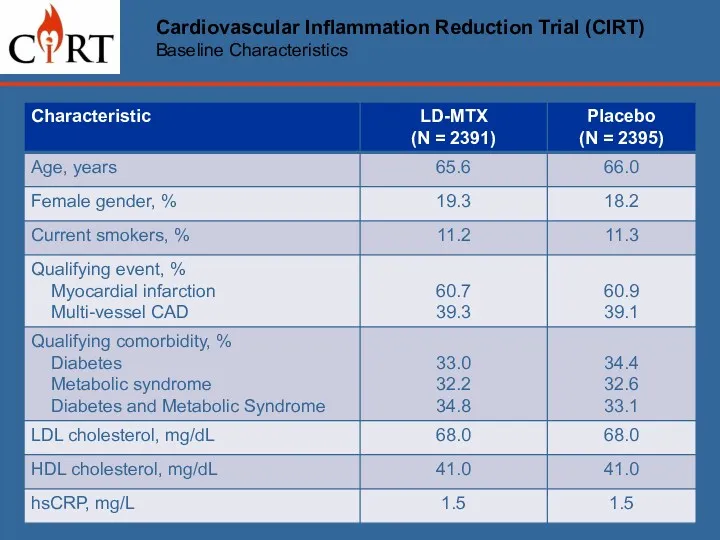
- 11. Cardiovascular Inflammation Reduction Trial (CIRT) Baseline Characteristics
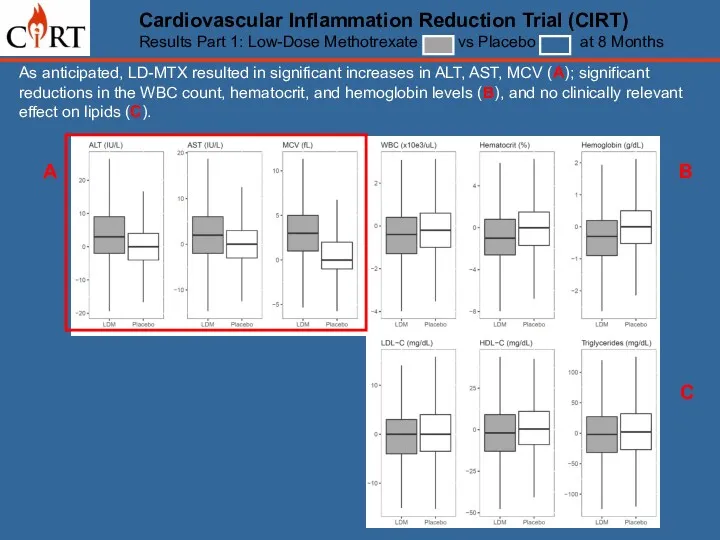
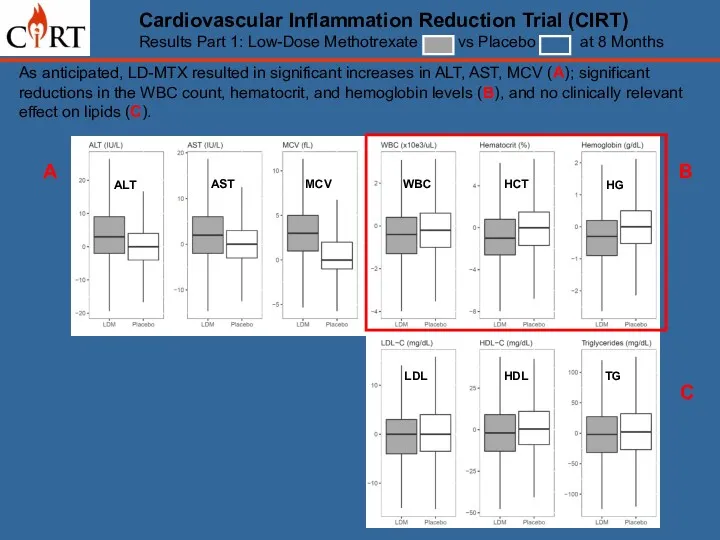
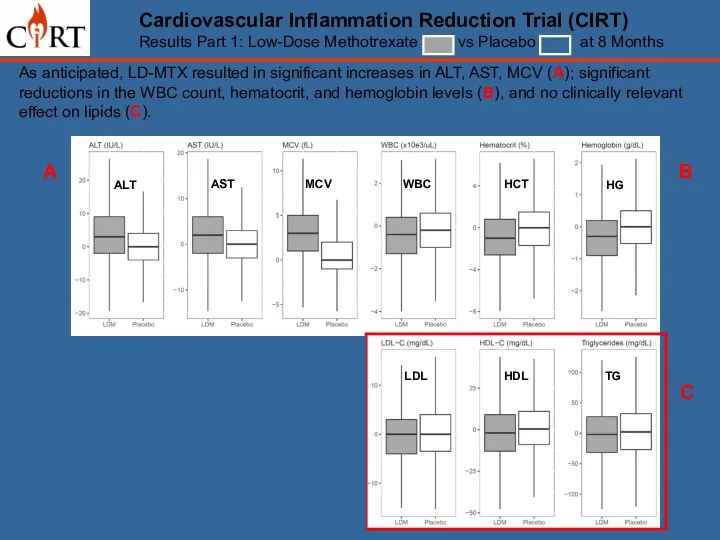
- 12. Cardiovascular Inflammation Reduction Trial (CIRT) Results Part 1: Low-Dose Methotrexate vs Placebo at 8 Months As
- 13. As anticipated, LD-MTX resulted in significant increases in ALT, AST, MCV (A); significant reductions in the
- 14. As anticipated, LD-MTX resulted in significant increases in ALT, AST, MCV (A); significant reductions in the
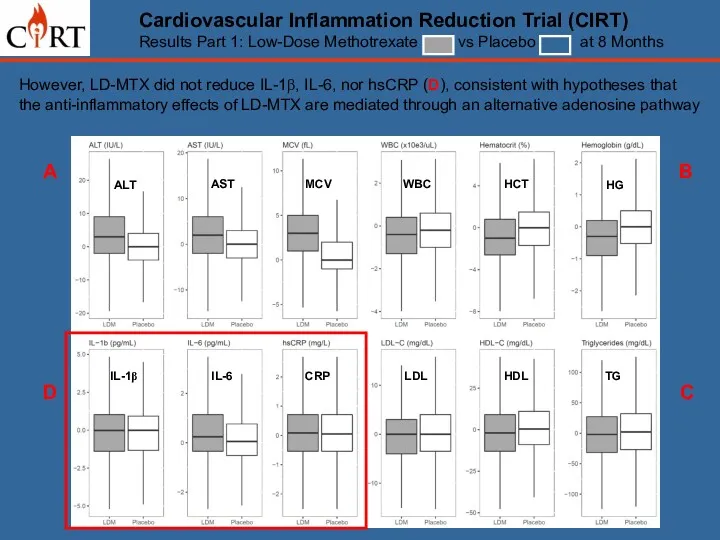
- 15. A B D C ALT AST MCV WBC HCT HG LDL HDL TG IL-1β IL-6 CRP
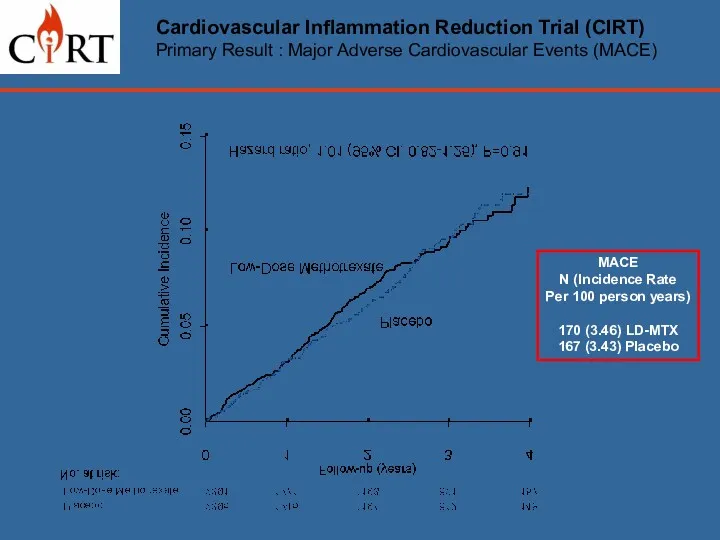
- 16. Cardiovascular Inflammation Reduction Trial (CIRT) Primary Result : Major Adverse Cardiovascular Events (MACE) MACE N (Incidence
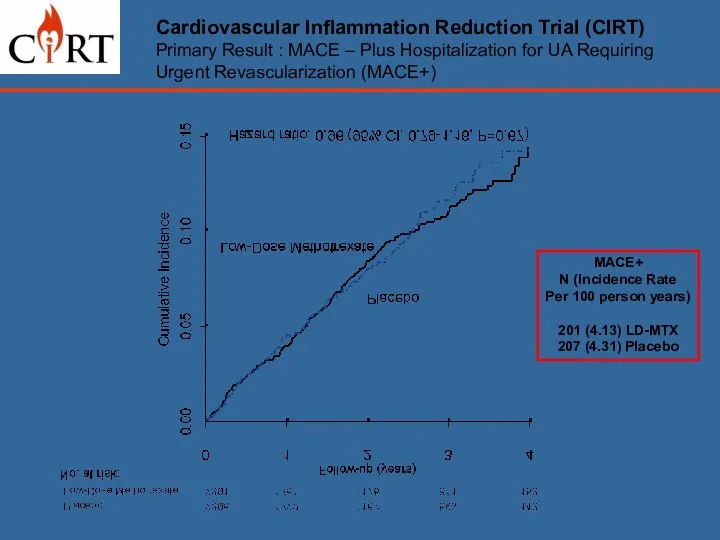
- 17. Cardiovascular Inflammation Reduction Trial (CIRT) Primary Result : MACE – Plus Hospitalization for UA Requiring Urgent
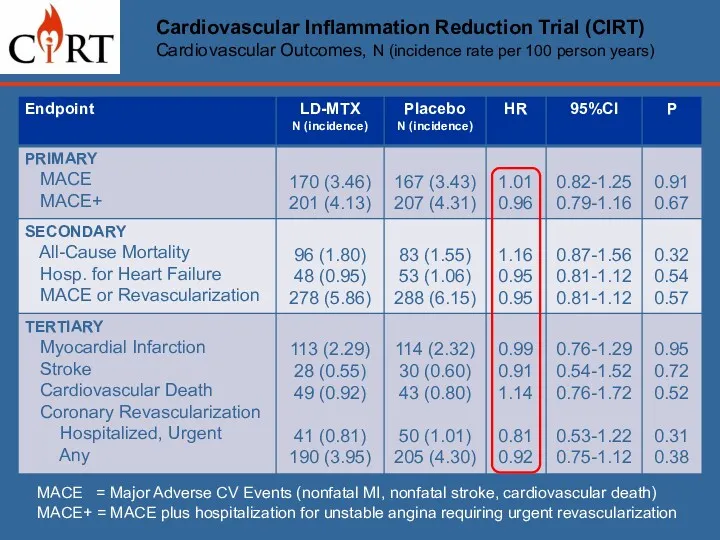
- 18. Cardiovascular Inflammation Reduction Trial (CIRT) Cardiovascular Outcomes, N (incidence rate per 100 person years) MACE =
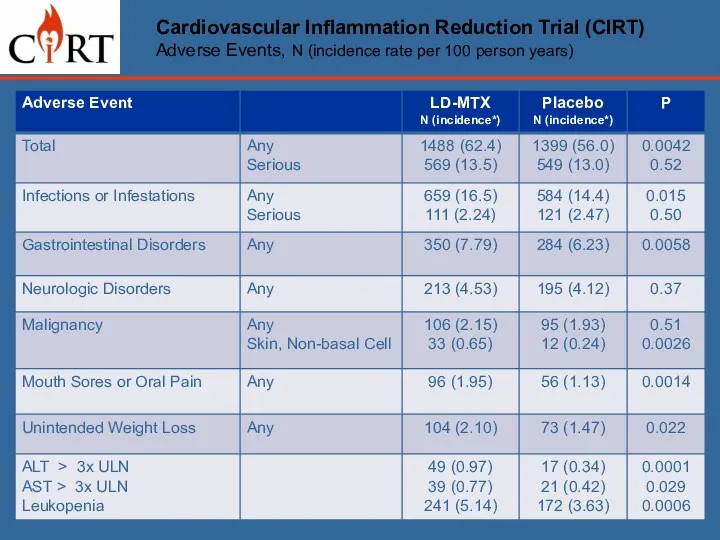
- 19. Cardiovascular Inflammation Reduction Trial (CIRT) Adverse Events, N (incidence rate per 100 person years)
- 20. Interleukin-1β Inhibition IL-1β IL-6 hsCRP 17% reduction in MACE+ Low-Dose Methotrexate IL-1β IL-6 hsCRP No reduction
- 21. Taken together, the CANTOS and CIRT trials demonstrate that inflammation inhibition can significantly reduce cardiovascular event
- 23. Скачать презентацию



 Гипертензивные состояния. Тема 4
Гипертензивные состояния. Тема 4 Ιστολογία ΙΙ – Κυτταρολογία (Θ). Πεπτικοί Αδένες
Ιστολογία ΙΙ – Κυτταρολογία (Θ). Πεπτικοί Αδένες Кесарево сечение в современном акушерстве
Кесарево сечение в современном акушерстве Анестезия в нейрохирургии
Анестезия в нейрохирургии Анатомия, физиология кожи. Морфологические элементы кожных сыпей
Анатомия, физиология кожи. Морфологические элементы кожных сыпей 6.زواج-القاصرات
6.زواج-القاصرات Абдоминальный болевой синдром
Абдоминальный болевой синдром Местная анестезия
Местная анестезия Результаты сравнительного постмаркетингового исследования комбинированных антиретровирусных препаратов
Результаты сравнительного постмаркетингового исследования комбинированных антиретровирусных препаратов Дифференциальная диагностика и поиск непосредственной причины смерти в поздний посттравматический период
Дифференциальная диагностика и поиск непосредственной причины смерти в поздний посттравматический период Что надо знать об артериальной гипертонии (Занятие 1)
Что надо знать об артериальной гипертонии (Занятие 1) Отделение медицинской реабилитации взрослых для пациентов с соматическими заболеваниями
Отделение медицинской реабилитации взрослых для пациентов с соматическими заболеваниями СП при энтеритах
СП при энтеритах Предсердные нарушения ритма
Предсердные нарушения ритма Ткани челюстно-лицевой области
Ткани челюстно-лицевой области Рвотные и противорвотные средства
Рвотные и противорвотные средства Внезапная сердечная смерть у детей и подростков
Внезапная сердечная смерть у детей и подростков Физиологические роды
Физиологические роды Мерцательная аритмия
Мерцательная аритмия Диспансеризация населения
Диспансеризация населения Intoxicatiile acute cu ciuperci
Intoxicatiile acute cu ciuperci Жедел және созылмалы гломерулонефриттердің емі
Жедел және созылмалы гломерулонефриттердің емі Методы исследования сердечной деятельности
Методы исследования сердечной деятельности Несостоятельность мышц тазового дна. Основные методы лечения
Несостоятельность мышц тазового дна. Основные методы лечения Понятие о химиотерапии и антибиотики
Понятие о химиотерапии и антибиотики Перспективы внедрения фармакогенетических подходов в клиническую практику
Перспективы внедрения фармакогенетических подходов в клиническую практику Лекция. Пищеварение в тонком кишечнике
Лекция. Пищеварение в тонком кишечнике Орталық нерв жүйесі
Орталық нерв жүйесі