Содержание
- 2. Case 20 YO male Admitted to the Neurology department on April, 11th, 2016 Complaining of gait
- 3. History of present illness 2010 – sudden vertigo, horizontal diplopia, oculomotor abnormalities, gait ataxia. He was
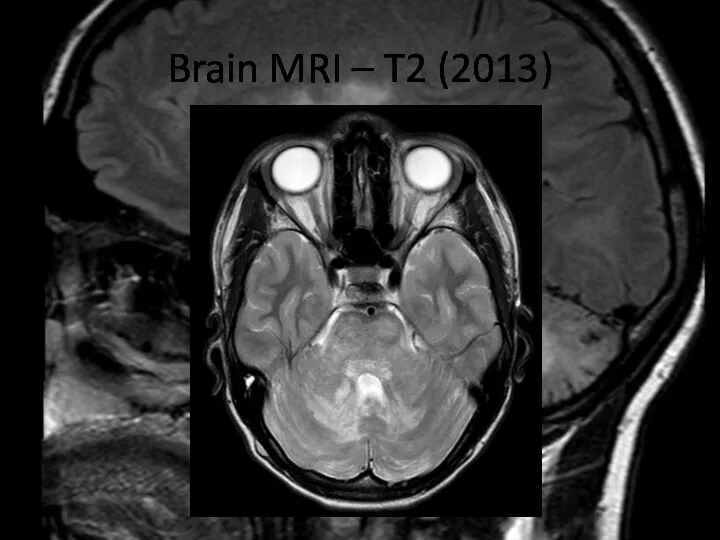
- 4. Brain MRI – T2 (2013)
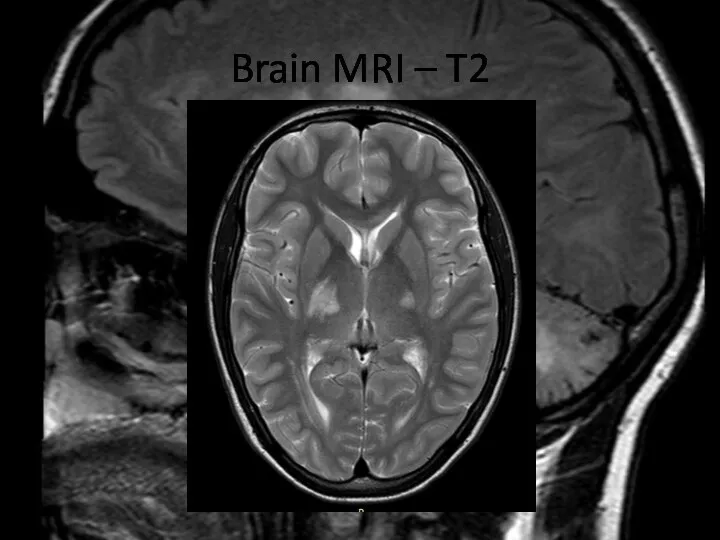
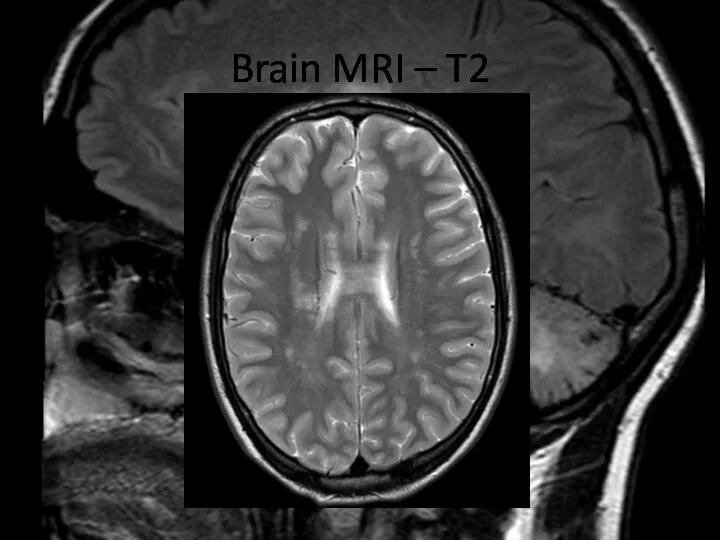
- 5. Brain MRI – T2
- 6. Brain MRI – T2
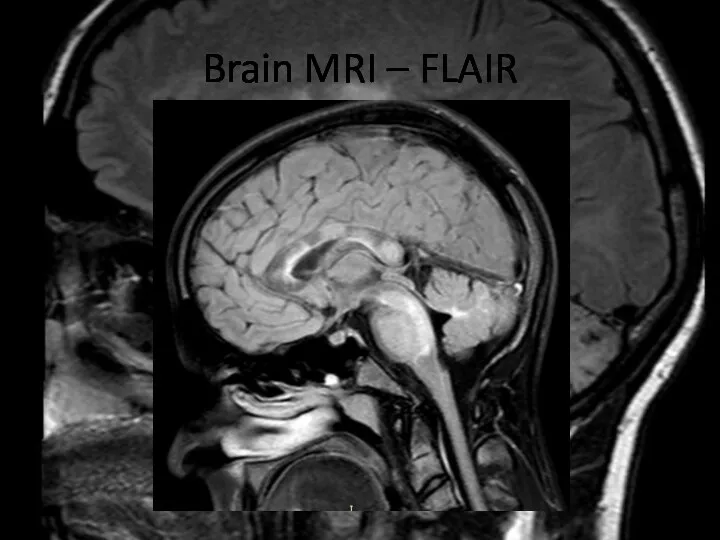
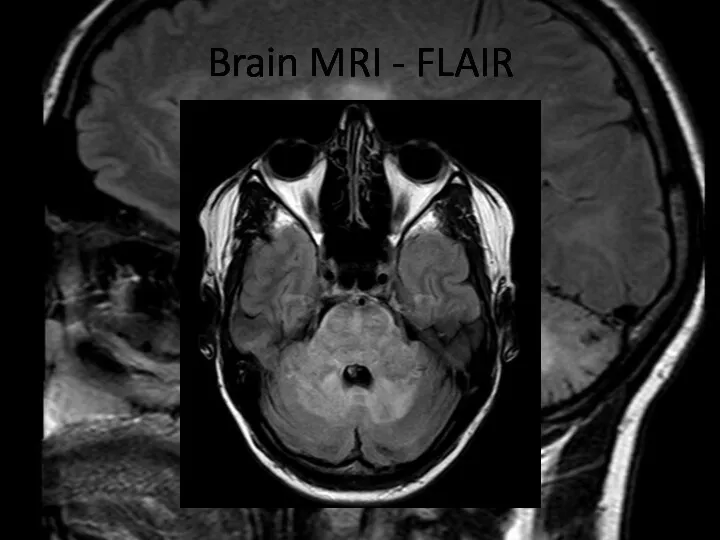
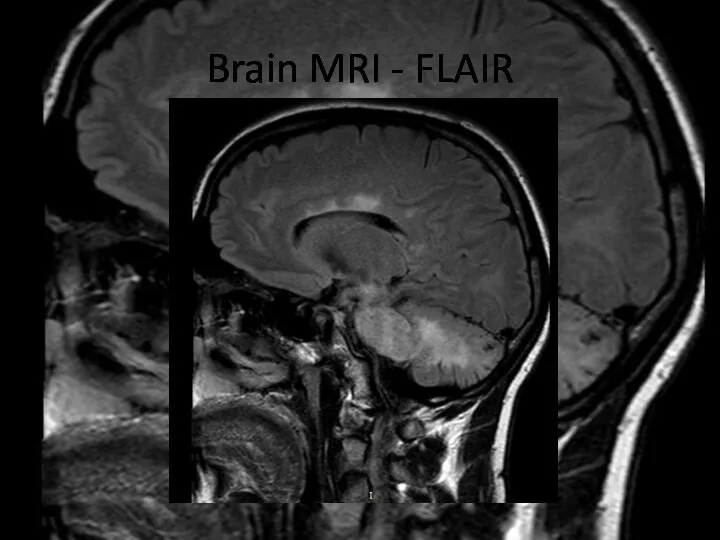
- 7. Brain MRI – FLAIR
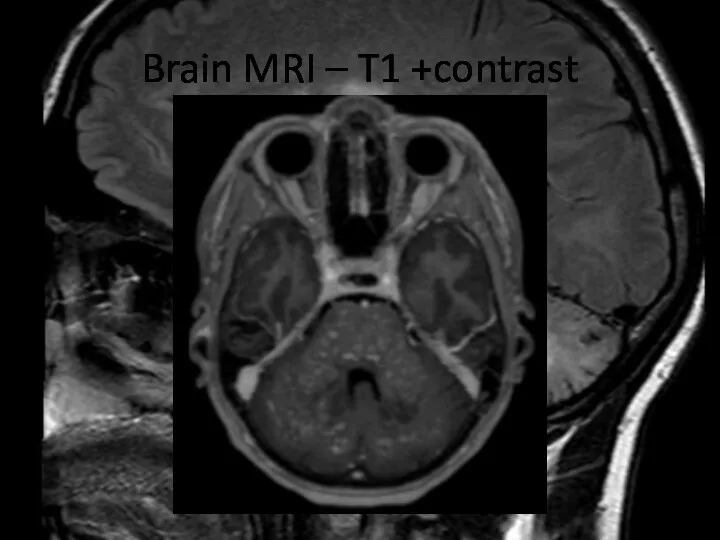
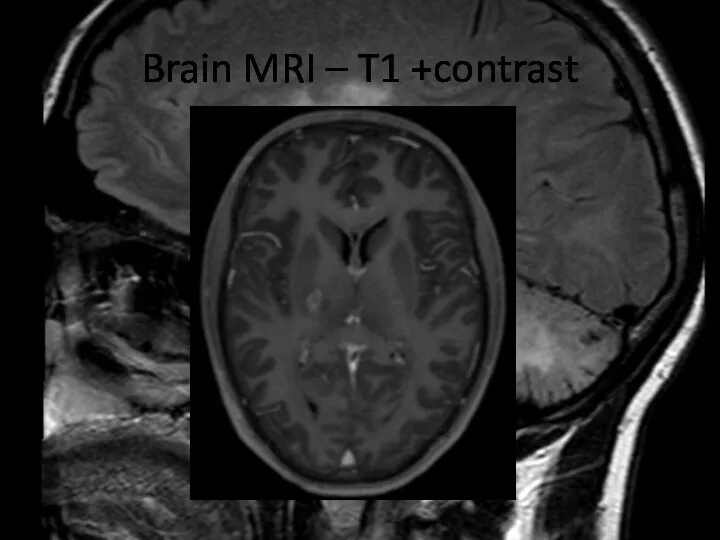
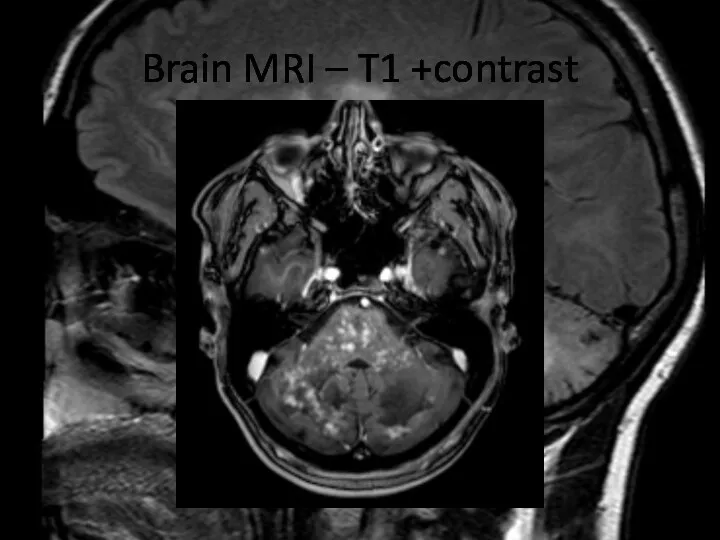
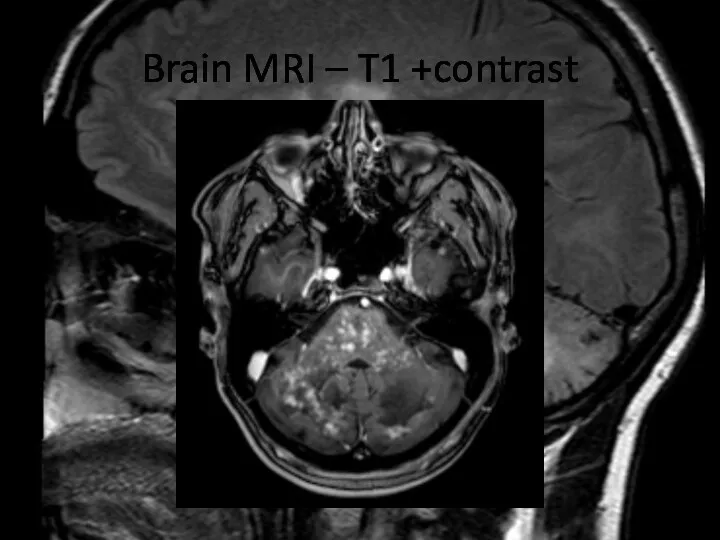
- 8. Brain MRI – T1 +contrast
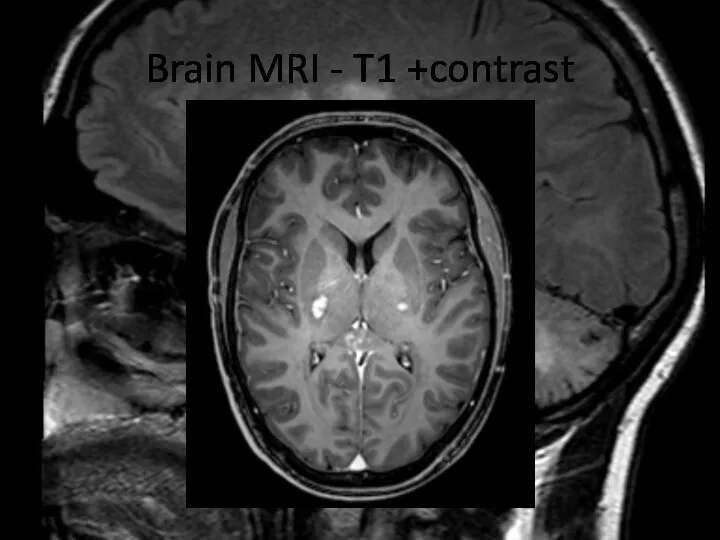
- 9. Brain MRI – T1 +contrast
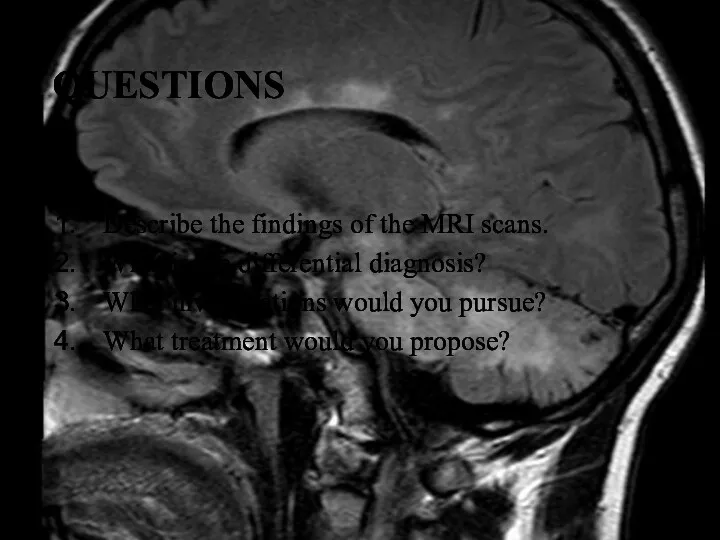
- 10. QUESTIONS Describe the findings of the MRI scans. What is the differential diagnosis? What investigations would
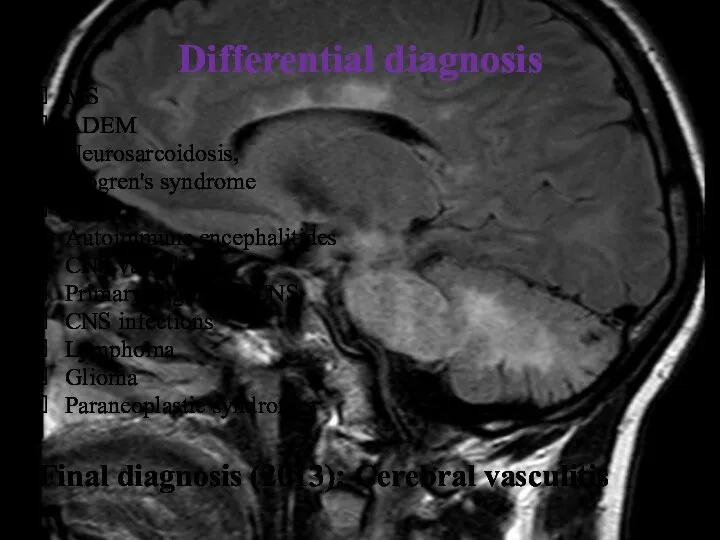
- 11. Differential diagnosis MS ADEM Neurosarcoidosis, Sjögren's syndrome NMO Autoimmune encephalitides CNS vasculitis Primary angiitis of CNS
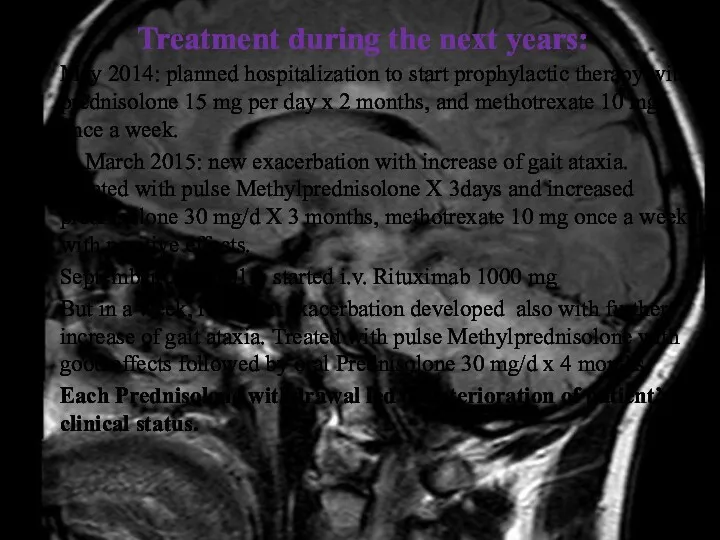
- 12. Treatment during the next years: May 2014: planned hospitalization to start prophylactic therapy with prednisolone 15
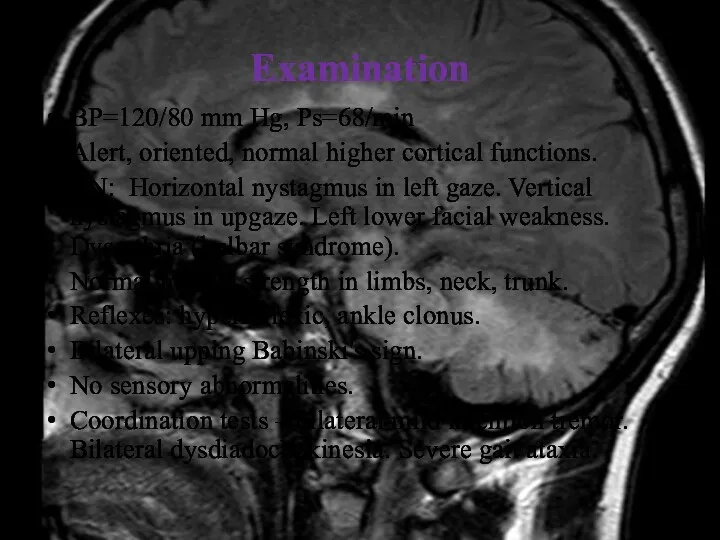
- 13. Examination BP=120/80 mm Hg, Ps=68/min Alert, oriented, normal higher cortical functions. CN: Horizontal nystagmus in left
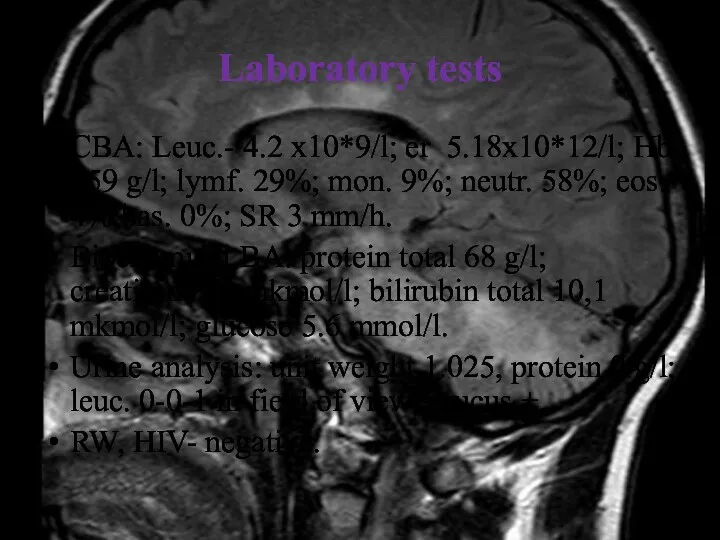
- 14. Laboratory tests CBA: Leuc.- 4.2 x10*9/l; er 5.18x10*12/l; Hb 159 g/l; lymf. 29%; mon. 9%; neutr.
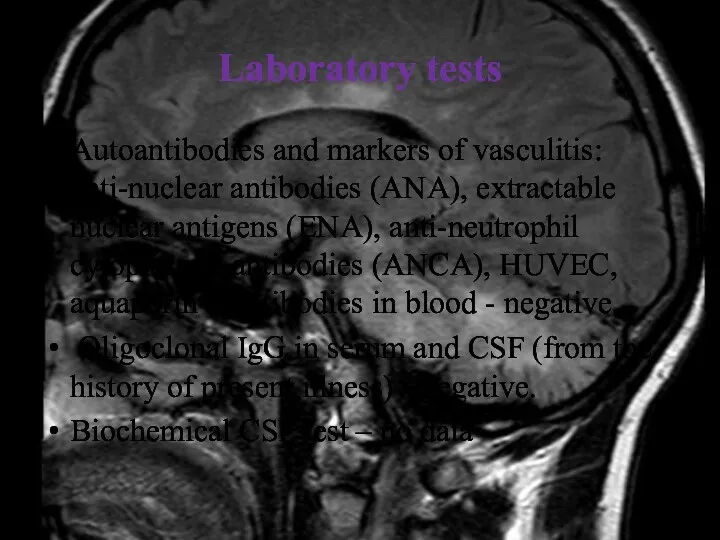
- 15. Laboratory tests Autoantibodies and markers of vasculitis: anti-nuclear antibodies (ANA), extractable nuclear antigens (ENA), anti-neutrophil cytoplasmic
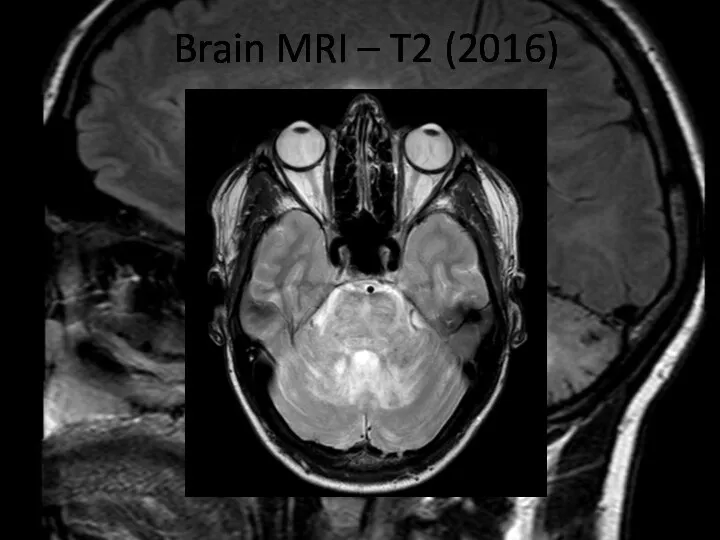
- 16. Brain MRI – T2 (2016)
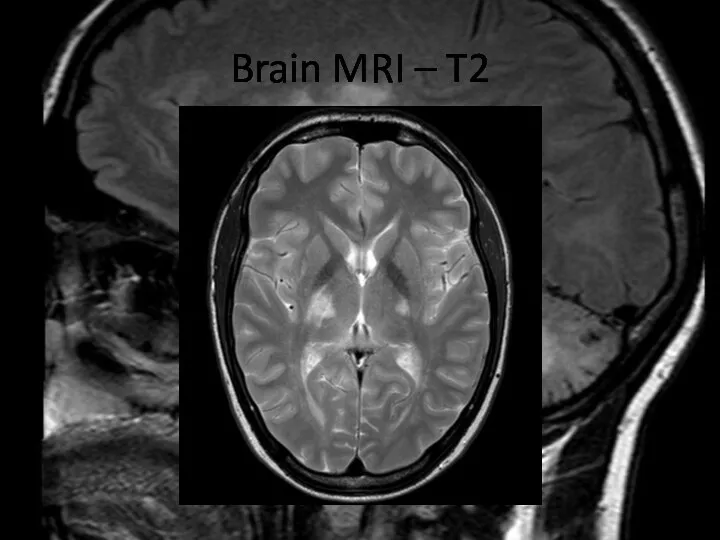
- 17. Brain MRI – T2
- 18. Brain MRI - FLAIR
- 19. Brain MRI - FLAIR
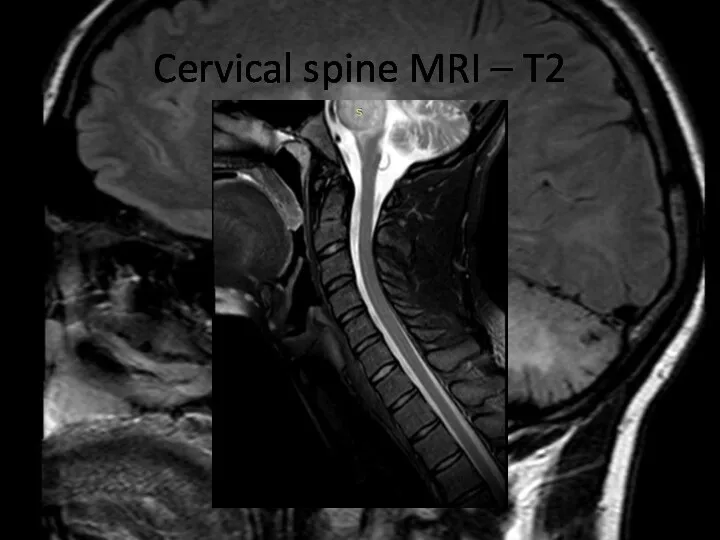
- 20. Cervical spine MRI – T2
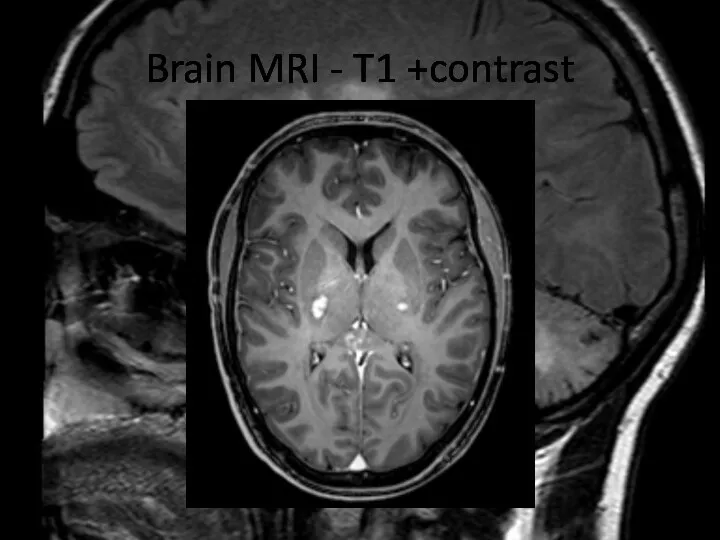
- 21. Brain MRI – T1 +contrast
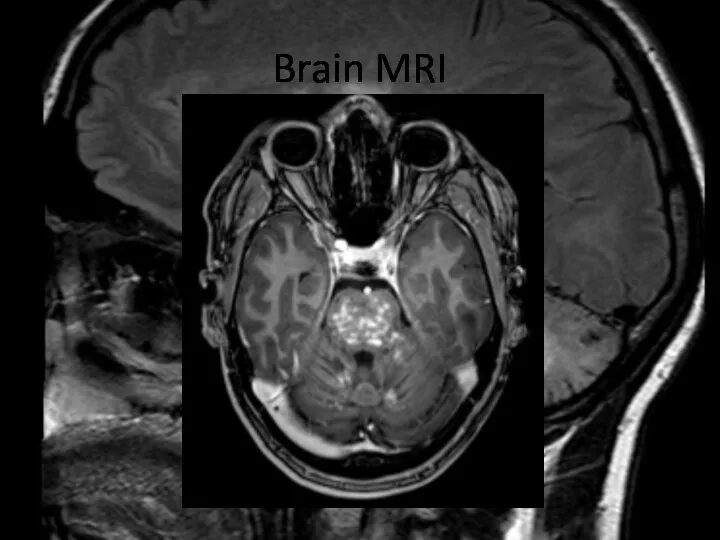
- 22. Brain MRI
- 23. Brain MRI - T1 +contrast
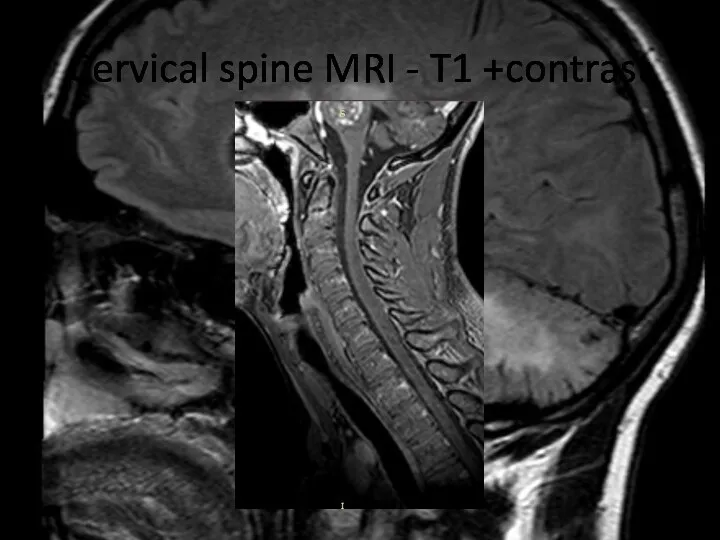
- 24. Cervical spine MRI - T1 +contrast
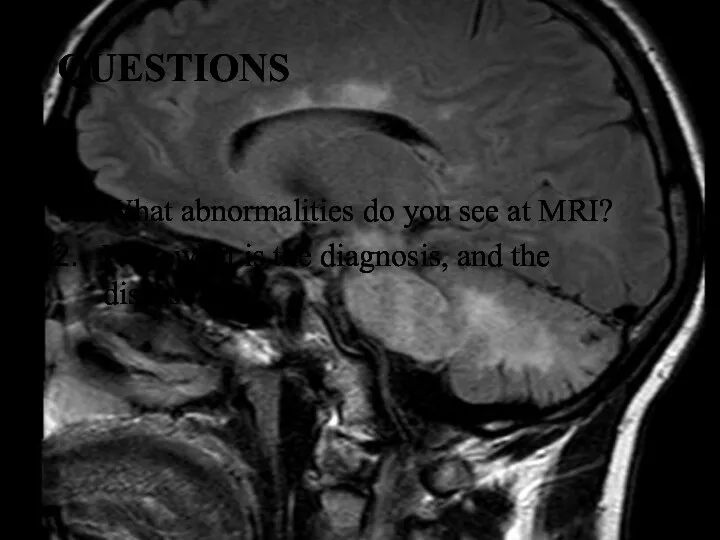
- 25. QUESTIONS What abnormalities do you see at MRI? Now what is the diagnosis, and the disease?
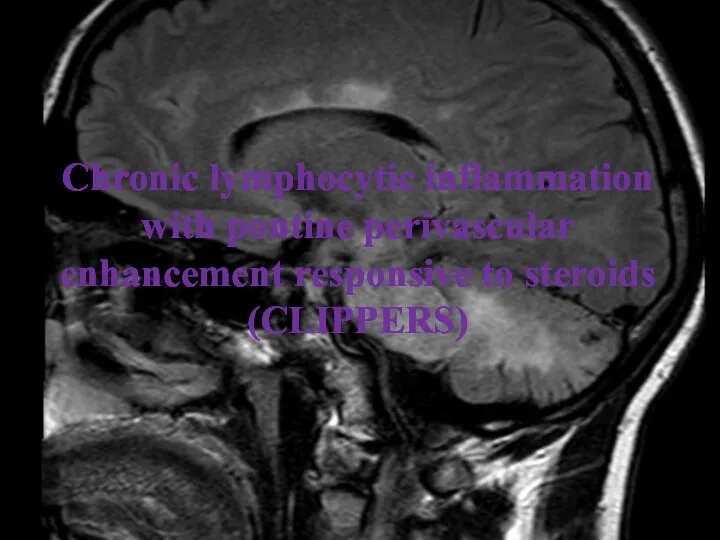
- 26. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS)
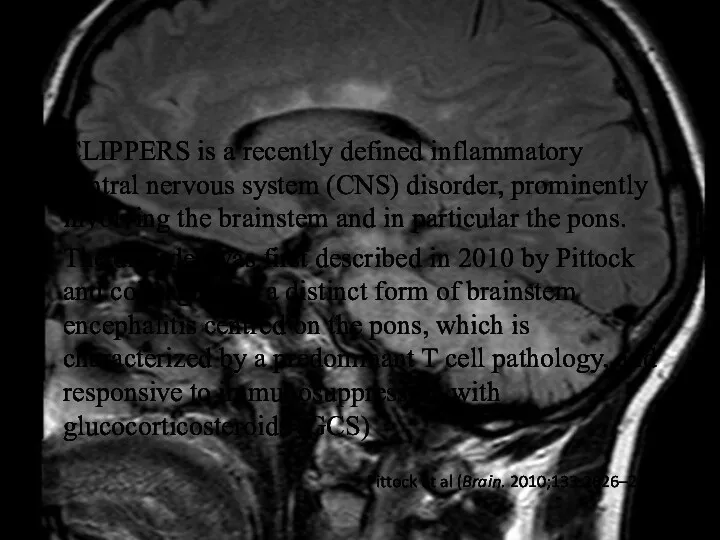
- 27. CLIPPERS is a recently defined inflammatory central nervous system (CNS) disorder, prominently involving the brainstem and
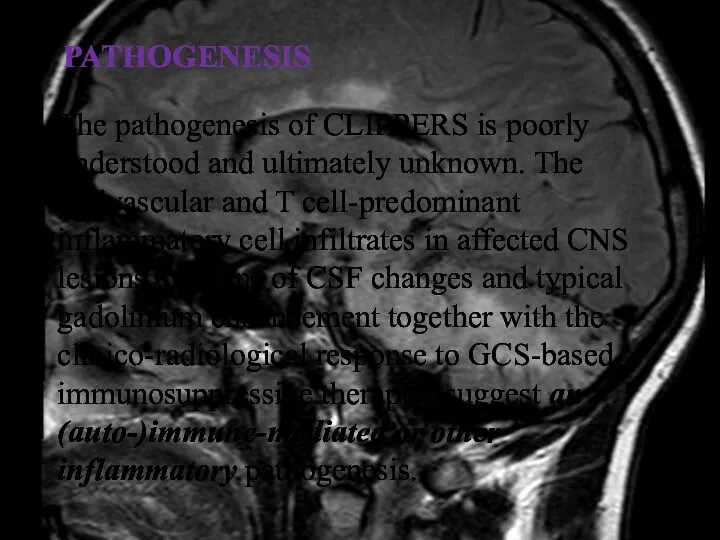
- 28. PATHOGENESIS The pathogenesis of CLIPPERS is poorly understood and ultimately unknown. The perivascular and T cell-predominant
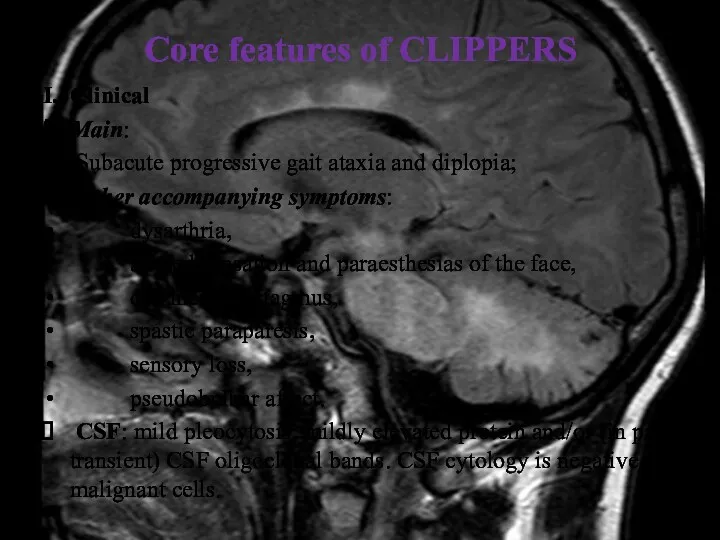
- 29. Core features of CLIPPERS I. Clinical Main: Subacute progressive gait ataxia and diplopia; Other accompanying symptoms:
- 30. Core features of CLIPPERS II. Radiological Numerous punctate or nodular enhancing lesions bilaterally within at least
- 31. Brain MRI – T1 +contrast
- 32. Brain MRI - T1 +contrast
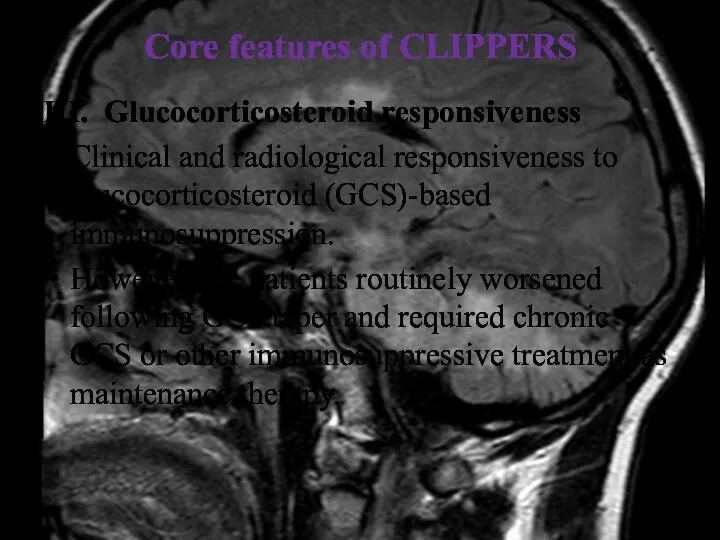
- 33. Core features of CLIPPERS III. Glucocorticosteroid responsiveness Clinical and radiological responsiveness to glucocorticosteroid (GCS)-based immunosuppression. However,
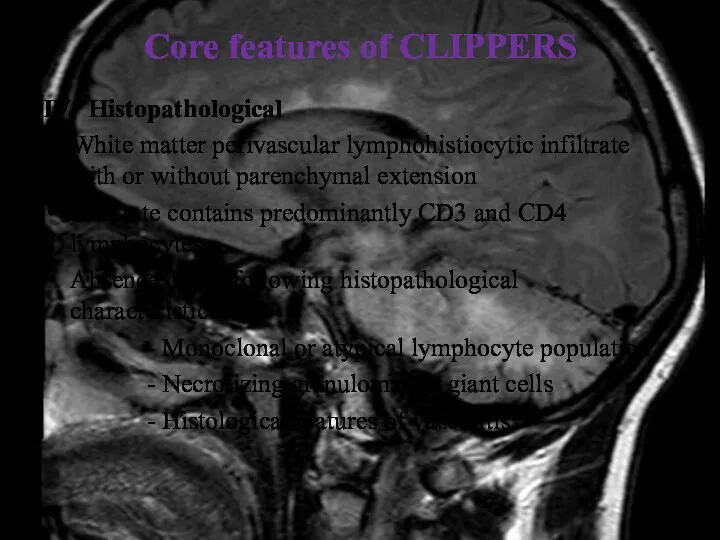
- 34. Core features of CLIPPERS IV. Histopathological White matter perivascular lymphohistiocytic infiltrate with or without parenchymal extension
- 35. Core features of CLIPPERS Differential diagnoses should be excluded e.g. neurosarcoidosis, Sjögren's syndrome, neuro-Behçet's disease, MS,
- 36. «Red flags» No response to treatment with GCS at the beginning or during follow-up. Unusual clinical
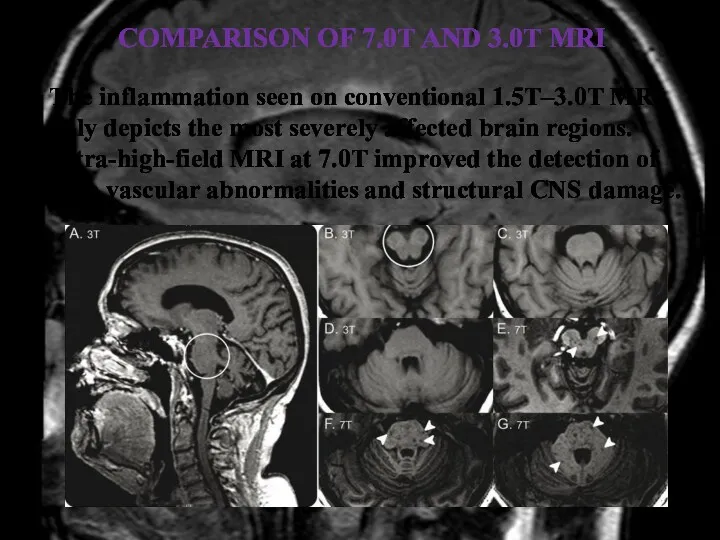
- 37. COMPARISON OF 7.0T AND 3.0T MRI The inflammation seen on conventional 1.5T–3.0T MRI only depicts the
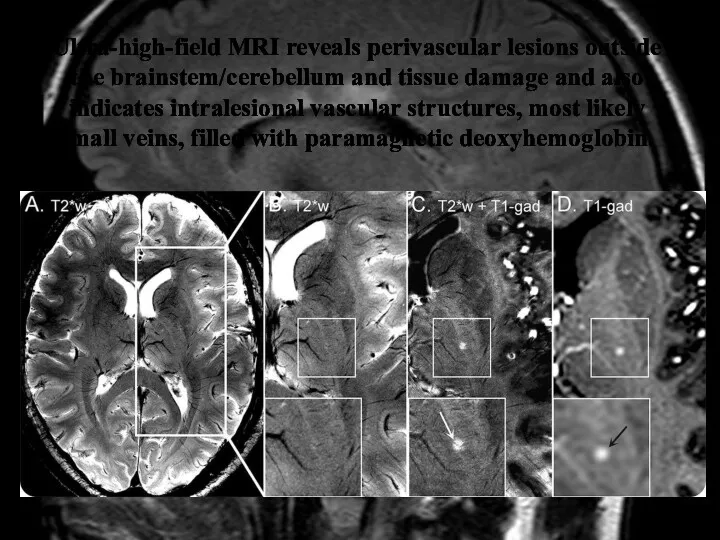
- 38. Ultra-high-field MRI reveals perivascular lesions outside the brainstem/cerebellum and tissue damage and also indicates intralesional vascular
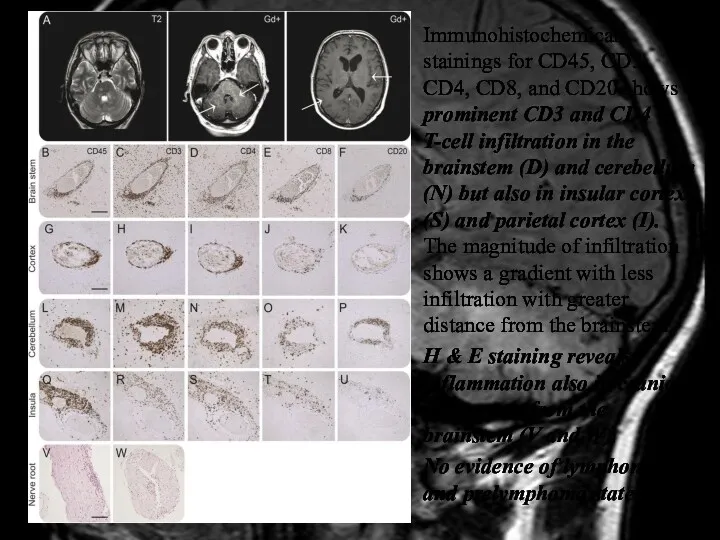
- 39. Immunohistochemical stainings for CD45, CD3, CD4, CD8, and CD20 shows prominent CD3 and CD4 T-cell infiltration
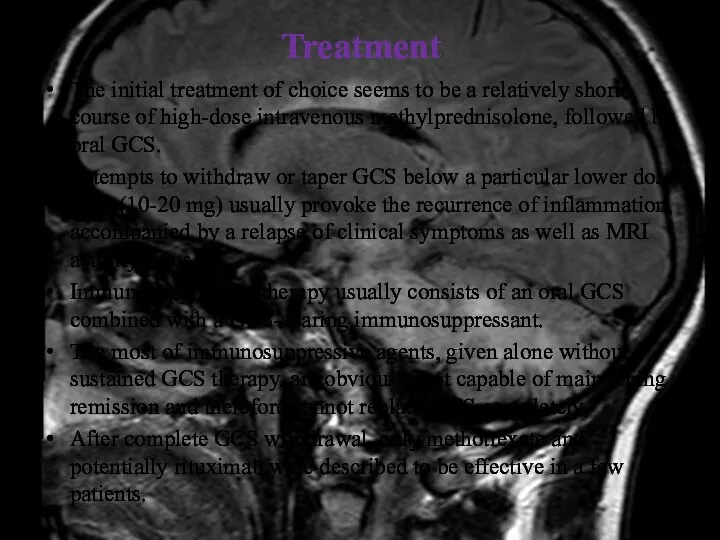
- 40. Treatment The initial treatment of choice seems to be a relatively short course of high-dose intravenous
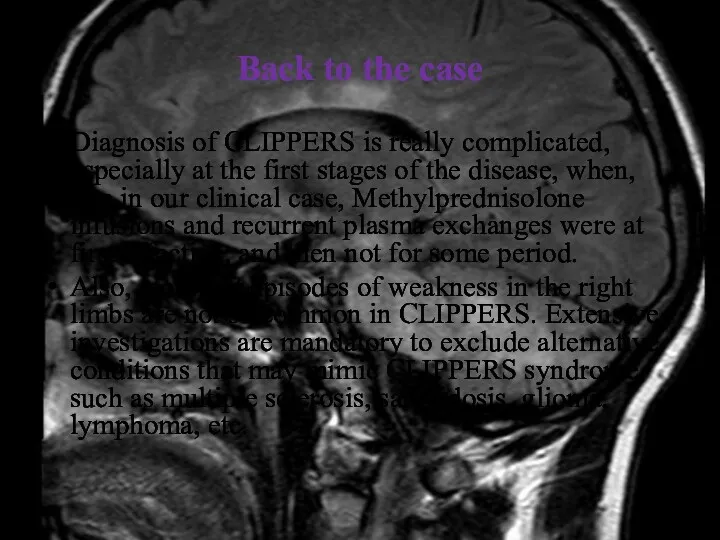
- 41. Back to the case Diagnosis of CLIPPERS is really complicated, especially at the first stages of
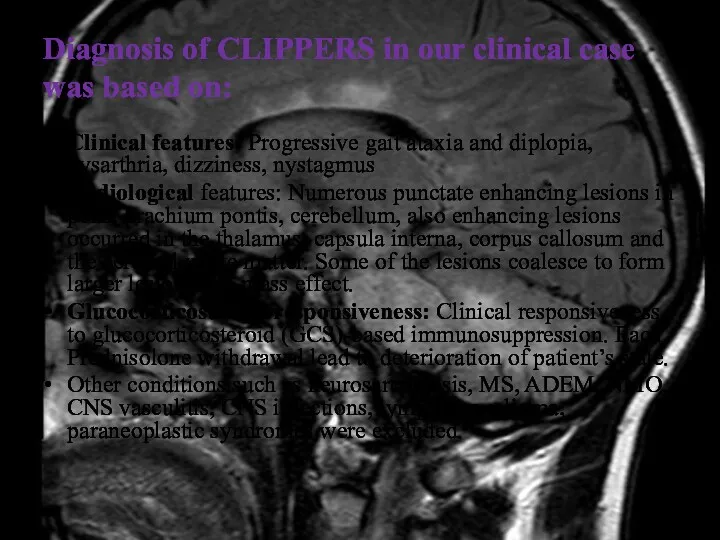
- 42. Diagnosis of CLIPPERS in our clinical case was based on: Clinical features: Progressive gait ataxia and
- 43. CONCLUSION Diagnosis of CLIPPERS is challenging, and requires careful exclusion of alternative diagnoses. A specific serum
- 44. References: Buttmann M, Metz I, Brecht I, Brück W, Warmuth-Metz M. Atypical chronic lymphocytic inflammation with
- 45. References: Tallantyre EC, Brookes MJ, Dixon JE, Morgan PS, Evangelou N, Morris PG. Demonstrating the perivascular
- 47. Скачать презентацию


 ЛС, применяемые в аллергологии
ЛС, применяемые в аллергологии Гигиена детских и образовательных учреждений
Гигиена детских и образовательных учреждений Пневмония. Қауіп қатер тобы. Клиникасы. Диагностикасы
Пневмония. Қауіп қатер тобы. Клиникасы. Диагностикасы Санаторий Магистраль
Санаторий Магистраль Fizjoterapia oddechowa
Fizjoterapia oddechowa Черепно–мозговая травма. Классификация. Система желудочков мозга
Черепно–мозговая травма. Классификация. Система желудочков мозга Туберкульоз. Історія боротьби
Туберкульоз. Історія боротьби Электрокардиография. История ЭКГ
Электрокардиография. История ЭКГ Десмургия. Школа медицинских волонтеров
Десмургия. Школа медицинских волонтеров Эпидемиологический мониторинг распространённости факторов риска хронических неинфекционных заболеваний в Республике Коми
Эпидемиологический мониторинг распространённости факторов риска хронических неинфекционных заболеваний в Республике Коми Классификации нарушений речи у детей
Классификации нарушений речи у детей Эксперименталды зерттеулерді ұйымдастыру мен жүргізудің ерекшеліктері
Эксперименталды зерттеулерді ұйымдастыру мен жүргізудің ерекшеліктері Специфические показатели качества жидких, твердых, мягких и асептически приготовленных лекарственных средств
Специфические показатели качества жидких, твердых, мягких и асептически приготовленных лекарственных средств Противокашлевые средства
Противокашлевые средства Несостоятельность тазового дна
Несостоятельность тазового дна Тубулоинтерстициальные нефропатии
Тубулоинтерстициальные нефропатии Внимание родители!
Внимание родители! Лейкоз ауруы кезіндегі зертханалық әдістер
Лейкоз ауруы кезіндегі зертханалық әдістер Pharmaceutical monitoring and evaluation
Pharmaceutical monitoring and evaluation Психология детей с сенсорными нарушениями
Психология детей с сенсорными нарушениями Клещевой энцефалит и болезнь Лайма
Клещевой энцефалит и болезнь Лайма Аномалии родовой деятельности. Лекция 2
Аномалии родовой деятельности. Лекция 2 Переливание компонентов крови и плазмозамещающих растворов
Переливание компонентов крови и плазмозамещающих растворов Осложнения после удаления зуба
Осложнения после удаления зуба Патология гортани. Гортань (анатомо-физиологическая справка)
Патология гортани. Гортань (анатомо-физиологическая справка) Эпидемиологический подход к изучению болезней человека
Эпидемиологический подход к изучению болезней человека Жасуша патофизиологиясы
Жасуша патофизиологиясы Drugs used in endocrine disorders
Drugs used in endocrine disorders