Содержание
- 2. Topics Concepts on pharmaceutical assessment/monitoring The WHO process on assessing and monitoring pharmaceutical situation Undertaking survey,
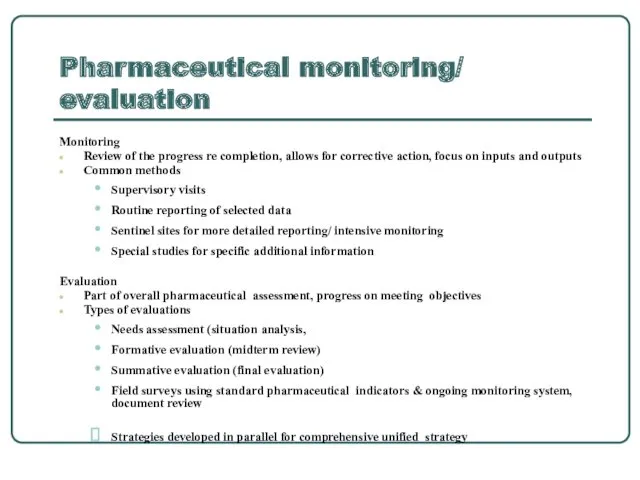
- 3. Pharmaceutical monitoring/ evaluation Monitoring Review of the progress re completion, allows for corrective action, focus on
- 4. Who can use the results from assessment and monitoring? Countries - focus action, prioritize, measure achievement
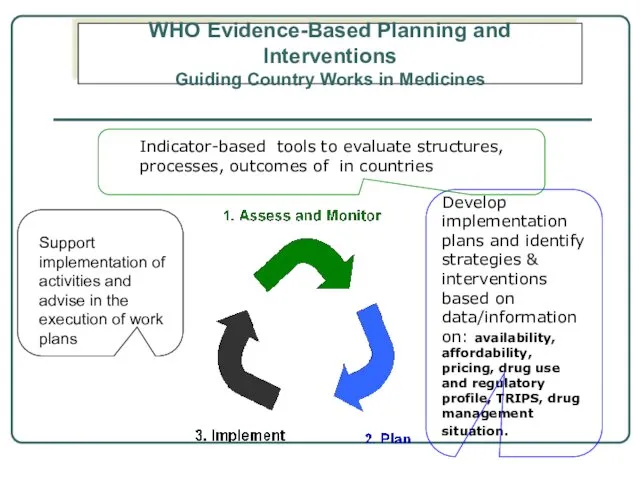
- 5. Develop implementation plans and identify strategies & interventions based on data/information on: availability, affordability, pricing, drug
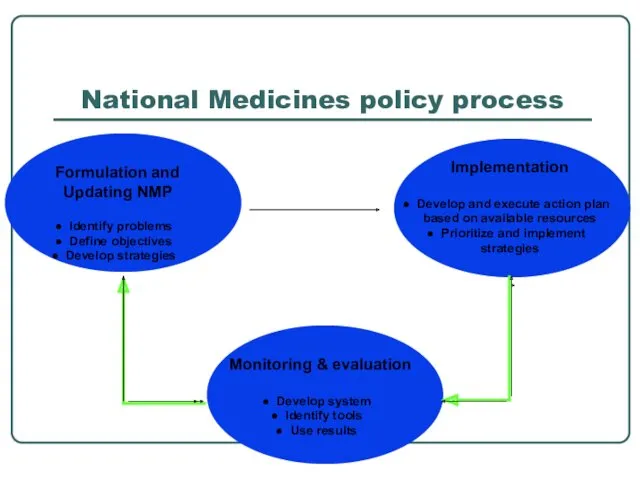
- 6. National Medicines policy process
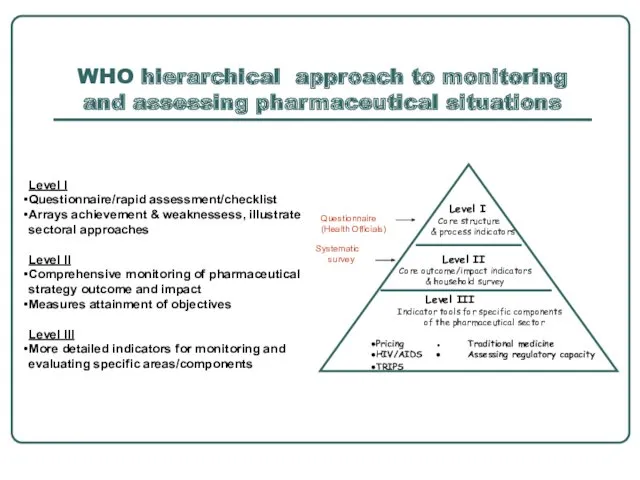
- 7. WHO hierarchical approach to monitoring and assessing pharmaceutical situations Level I Core structure & process indicators
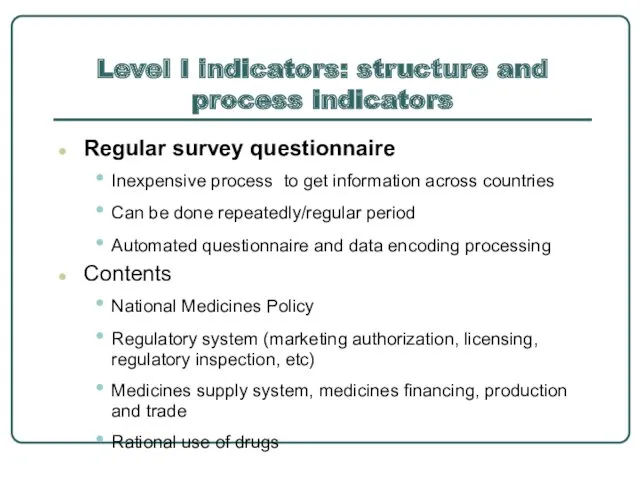
- 8. Level I indicators: structure and process indicators Regular survey questionnaire Inexpensive process to get information across
- 9. Level II- facility outcome and impactindicators: WHO Operational Package for Monitoring and Assessing County Pharmaceutical Situations"
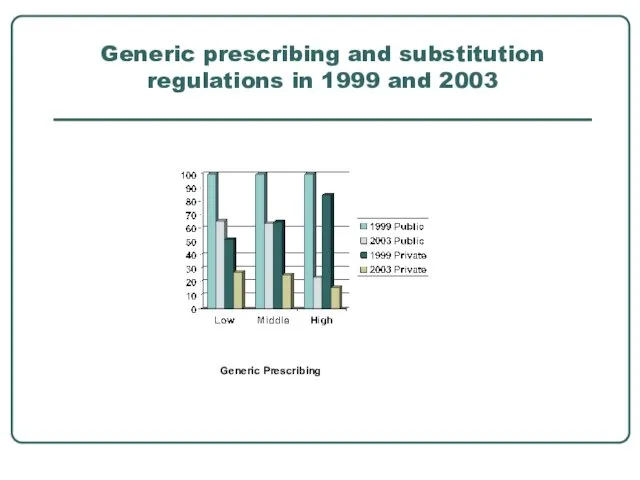
- 10. Generic prescribing and substitution regulations in 1999 and 2003 Generic Prescribing
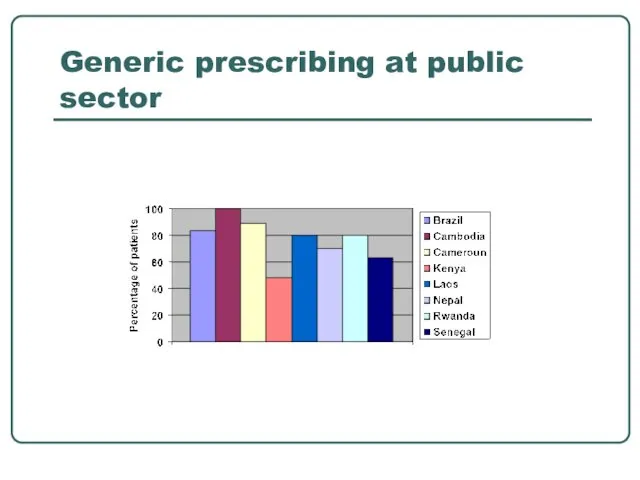
- 11. Generic prescribing at public sector
- 12. Measuring access to essential medicines ( Household Survey) Level I and Level II- facility surveys do
- 13. Importance of household survey Household situations How they access their medicines, where they get them How
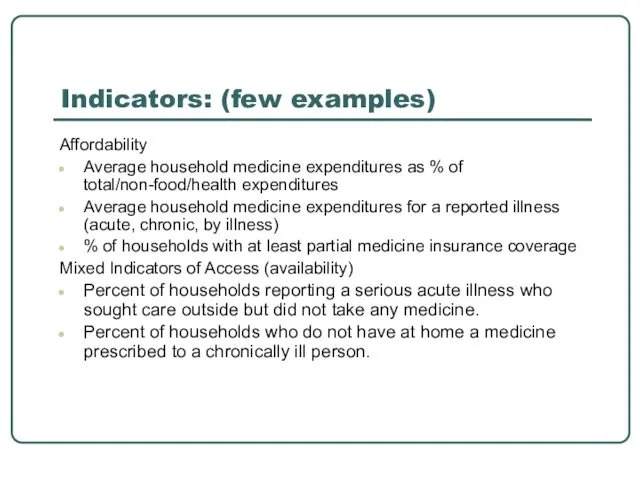
- 14. Indicators: (few examples) Affordability Average household medicine expenditures as % of total/non-food/health expenditures Average household medicine
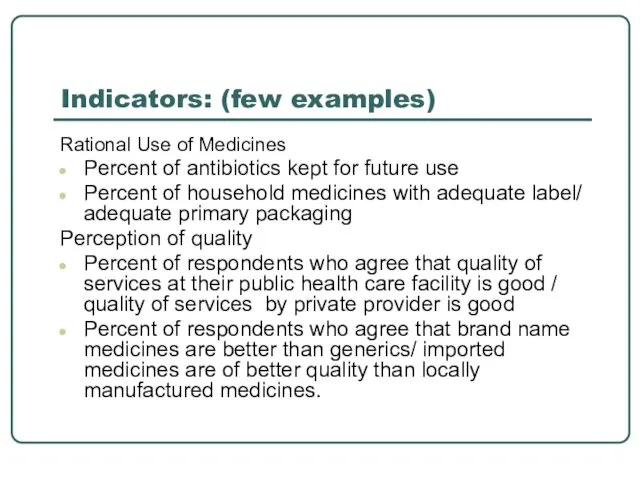
- 15. Indicators: (few examples) Rational Use of Medicines Percent of antibiotics kept for future use Percent of
- 16. Current issues on household survey process Challenge to use population based data to policy evaluation, development
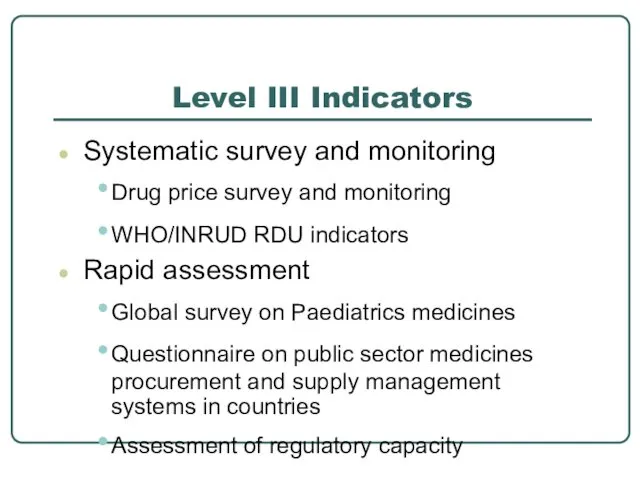
- 17. Level III Indicators Systematic survey and monitoring Drug price survey and monitoring WHO/INRUD RDU indicators Rapid
- 18. Sampling issues for systematic survey Follow specific procedures to minimize selection bias and is representative of
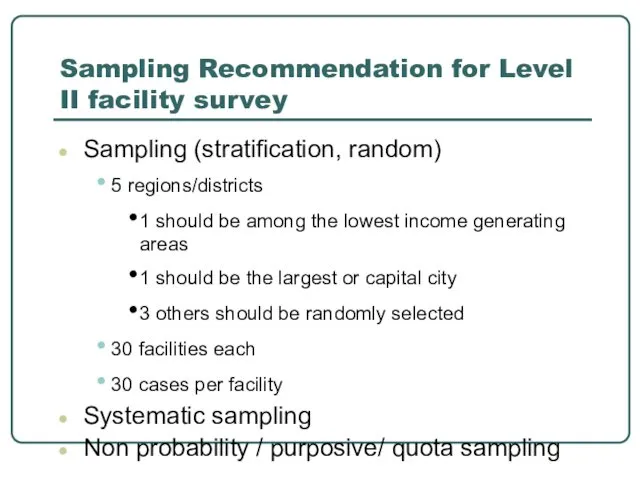
- 19. Sampling Recommendation for Level II facility survey Sampling (stratification, random) 5 regions/districts 1 should be among
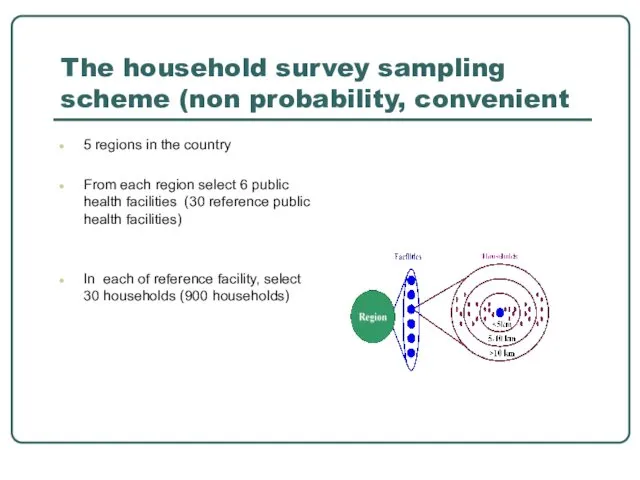
- 20. The household survey sampling scheme (non probability, convenient 5 regions in the country From each region
- 21. Is the sampling frame valid? (clustering in drug supply or drug use data) Geographic Characteristics Administration
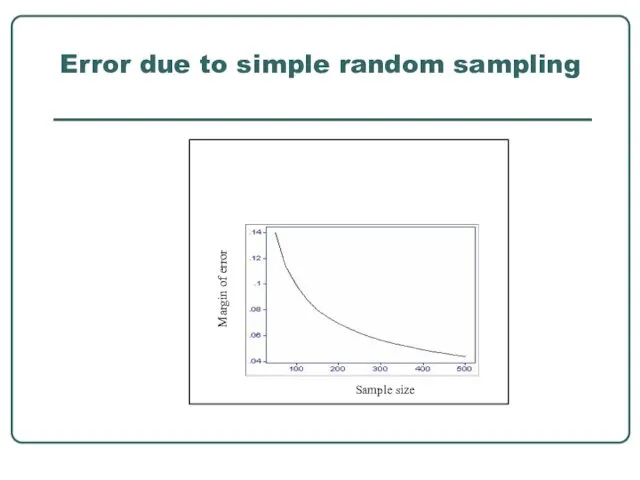
- 22. Error due to simple random sampling
- 23. Who can be trained to do the survey? Physicians, nurses, pharmacists or paramedical staff Health ministry/department
- 24. Preparing and implementing systematic survey Administrative preparation: Coordinating with WHO, ministry/department of health, public health facilities,
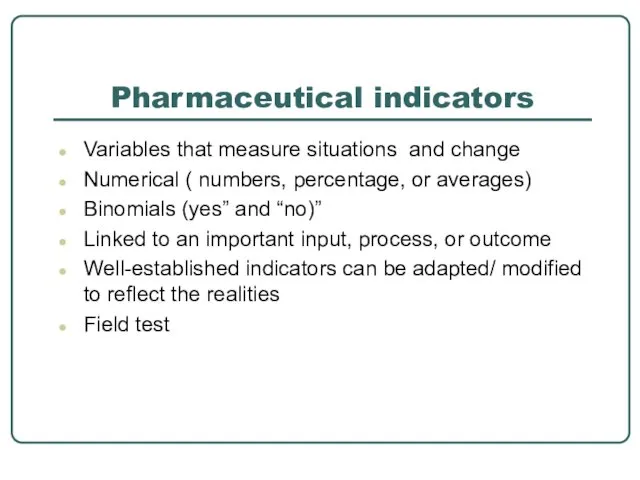
- 25. Pharmaceutical indicators Variables that measure situations and change Numerical ( numbers, percentage, or averages) Binomials (yes”
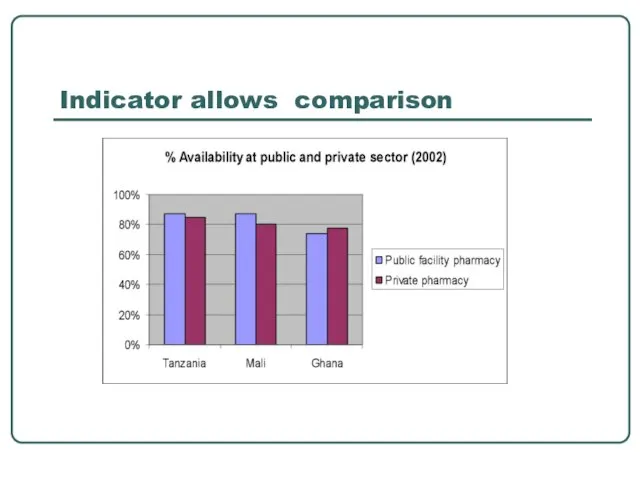
- 26. Why is it important to use indicators? Standard indicators facilitates: comparing the performance of facilities, districts,
- 27. Indicator allows comparison
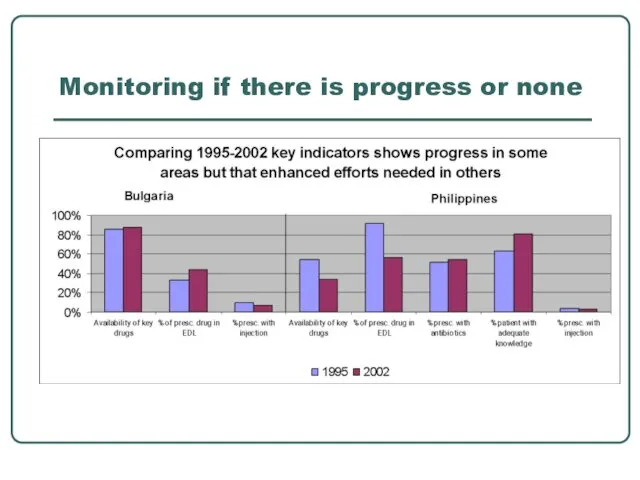
- 28. Monitoring if there is progress or none
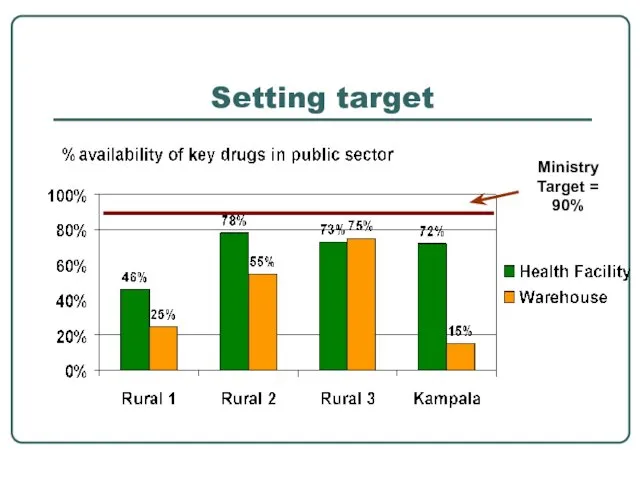
- 29. Setting target
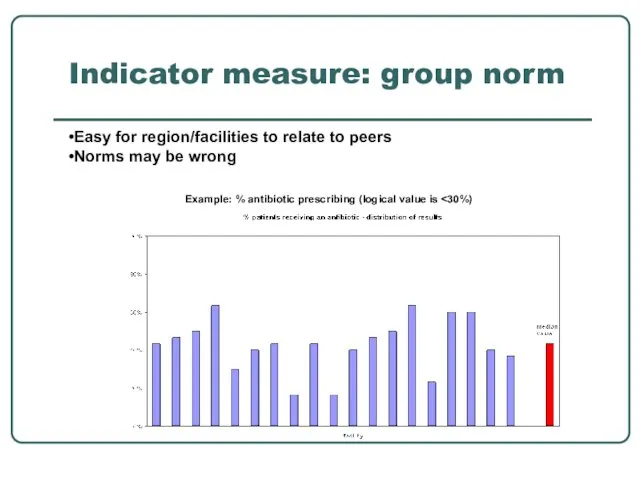
- 30. Indicator measure: group norm Example: % antibiotic prescribing (logical value is Easy for region/facilities to relate
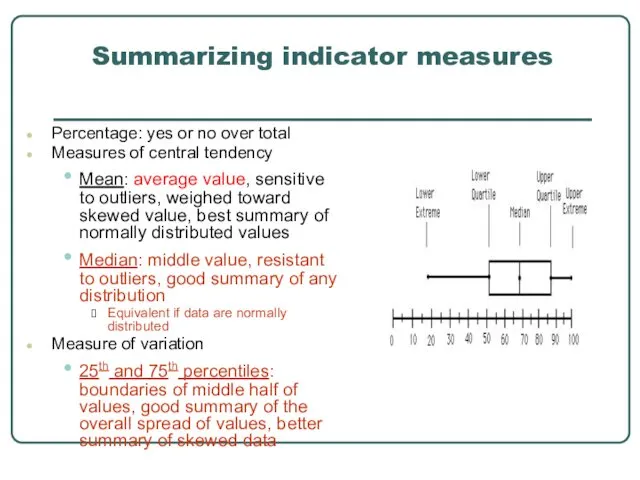
- 31. Summarizing indicator measures Percentage: yes or no over total Measures of central tendency Mean: average value,
- 32. Indicator measure: Ideal/logical values Logical value exist for some Logical value (100%-adequate labelling, meds dispensed, adherence
- 33. Connecting Survey Results and Interventions
- 34. The way forward on country monitoring Evidence through systematic but feasible data collection process is necessary
- 36. Скачать презентацию













 Акушерлік қанағулардың қауіп қатер факторын анықтау профилактика және реабилитация
Акушерлік қанағулардың қауіп қатер факторын анықтау профилактика және реабилитация Повреждение. Травма. Классификация. Виды травматизма. Клинические проявления и принципы лечения
Повреждение. Травма. Классификация. Виды травматизма. Клинические проявления и принципы лечения Қазақстан Республикасында сібір жарасы ауруының таралу корсеткіштері
Қазақстан Республикасында сібір жарасы ауруының таралу корсеткіштері Introduction to Rehabilitation
Introduction to Rehabilitation Взаимосвязь питания и хронических неинфекционных заболеваний
Взаимосвязь питания и хронических неинфекционных заболеваний Синдром Шерешевского-Тернера
Синдром Шерешевского-Тернера Форменные элементы крови
Форменные элементы крови Неотложная медицинская помощь и лечение при астматическом статусе
Неотложная медицинская помощь и лечение при астматическом статусе Қабыну процесі дегеніміз
Қабыну процесі дегеніміз Муковисцидоз. Этиология, патогенез. Диагностика. Емдеу әдістері
Муковисцидоз. Этиология, патогенез. Диагностика. Емдеу әдістері Патология печени
Патология печени Плацентарная недостаточность
Плацентарная недостаточность Эпидемиологическая ситуация заболеваемости корью. Алгоритм расследования побочных эффектов при вакцинации против кори
Эпидемиологическая ситуация заболеваемости корью. Алгоритм расследования побочных эффектов при вакцинации против кори Ожоги и рубцовые сужения пищевода у детей
Ожоги и рубцовые сужения пищевода у детей Цитостатические препараты
Цитостатические препараты Функциональная магнитно-резонансная томография
Функциональная магнитно-резонансная томография Гиподинамия как фактор риска развития заболеваний
Гиподинамия как фактор риска развития заболеваний Ишемическая болезнь сердца (ИБС). Стабильные формы
Ишемическая болезнь сердца (ИБС). Стабильные формы Результаты мониторинга начисления специальных социальных выплат
Результаты мониторинга начисления специальных социальных выплат Эндометрит
Эндометрит Нозология ғылымы
Нозология ғылымы Пороки развития гонад – дисгенезия гонад
Пороки развития гонад – дисгенезия гонад Визуальная диагностика дерматитов (контактно-аллергический, атопический, токсикодермический)
Визуальная диагностика дерматитов (контактно-аллергический, атопический, токсикодермический) Тістем және оның түрлері
Тістем және оның түрлері Пародонтологиядағы иммунотерапия
Пародонтологиядағы иммунотерапия Энтеровирусная инфекция сегодня
Энтеровирусная инфекция сегодня Доброкачественные опухоли желудка
Доброкачественные опухоли желудка Прикосновение ради здоровья. Программа по сохранению и улучшению здоровья
Прикосновение ради здоровья. Программа по сохранению и улучшению здоровья