Содержание
- 2. “Vector-BiAlgam”, PC The company was founded in 1996 on the ground of the State scientific center
- 3. Manufacturing base Probiotics manufacturing: Probiotics (supplements) Starters and technology for dairy industry Dry bacterial matter Bioproduct
- 4. Contract manufacturing The company “Vector-BiAlgam” provides contract manufacturing services in preparation and filling of injectable pharmaceuticals
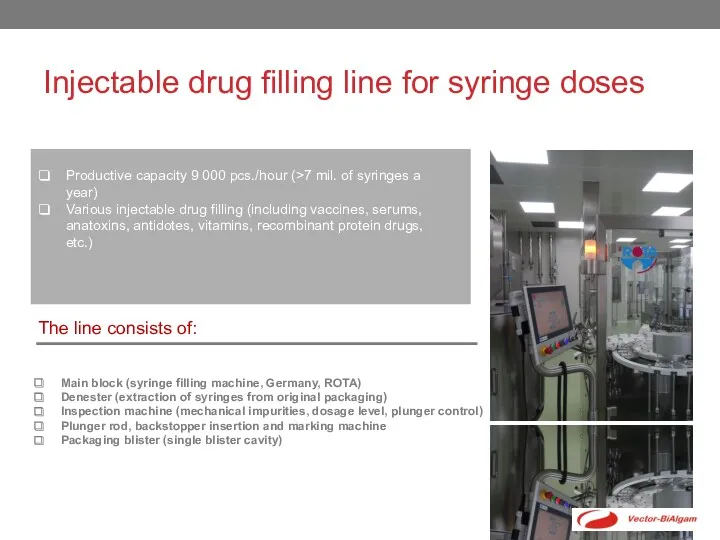
- 5. Injectable drug filling line for syringe doses Productive capacity 9 000 pcs./hour (>7 mil. of syringes
- 6. Combined ampoule and vial injectable drug filling line Productive capacity up to 9 000 ampoules/hour (>7
- 8. Скачать презентацию
“Vector-BiAlgam”, PC
The company was founded in 1996 on the ground of
“Vector-BiAlgam”, PC
The company was founded in 1996 on the ground of
Line of business:
manufacture of immunobiological pharmaceuticals and probiotics.
Leonid Nikulin
Chief executive director of “Vector-BiAlgam”, PC
Manufacturing base
Probiotics manufacturing:
Probiotics (supplements)
Starters and technology for dairy industry
Dry bacterial
Manufacturing base
Probiotics manufacturing:
Probiotics (supplements)
Starters and technology for dairy industry
Dry bacterial
Bioproduct
Probiotic micro-organism lysates
Injections manufacturing:
Hepatitis A vaccine “ALGAVAC® M”
(The product is made in form of a suspension of Hepatitis A virions (LBA-86 strain), purified, concentrated, inactivated, and adsorbed with aluminium hydroxide. One ml of the vaccine contains no less than 320 ELISA units of an antigen to Hepatitis A virus.)
GMP quality system
НАССР quality system
ISO quality management system
Contract manufacturing:
Preparation of injectables and filling of syringe doses
Preparation of injectables and filling of ampoules and vials.
Contract manufacturing
The company “Vector-BiAlgam” provides contract manufacturing services in preparation and
Contract manufacturing
The company “Vector-BiAlgam” provides contract manufacturing services in preparation and
Completion of the following operation cycle: preparation (manufacturing of the prepared product form from pharmaceutical substance), in-bulk filling, marking, packaging
Filling of products in full compliance with GMP standards
Full compliance with the technological cycle and quality control of finished product in accordance with the Client’s demands.
“Vector BiAlgam” guarantees:
Manufacturing filling area:
Injectable drug filling line for syringe doses (pre-filled syringes)
Combined ampoule/vial injectable drug filling line
Injectable drug filling line for syringe doses
Productive capacity 9 000 pcs./hour
Injectable drug filling line for syringe doses
Productive capacity 9 000 pcs./hour
Various injectable drug filling (including vaccines, serums, anatoxins, antidotes, vitamins, recombinant protein drugs, etc.)
The line consists of:
Main block (syringe filling machine, Germany, ROTA)
Denester (extraction of syringes from original packaging)
Inspection machine (mechanical impurities, dosage level, plunger control)
Plunger rod, backstopper insertion and marking machine
Packaging blister (single blister cavity)
Combined ampoule and vial injectable drug filling line
Productive capacity up to
Combined ampoule and vial injectable drug filling line
Productive capacity up to
Various injectable drug filling (including vaccines, serums, anatoxins, antidotes, vitamins, recombinant protein drugs, etc.)
The line consists of:
Washing machine for ampoules and vials (WR16)
Depyrogenization tunnel (DEPYR601) for sterilizing ampoules and vials
Filling and sealing machine for ampoules and vials RSF03
with capping system.
Automatic inspection machine (mechanical impurities, dosage level, integrity control) A35LD
Automatic labeling machine RL-F30




 Фитотерапия. Современная фитотерапия
Фитотерапия. Современная фитотерапия Ошибки и осложнения при лечении пострадавших с переломами костей и повреждениями суставов
Ошибки и осложнения при лечении пострадавших с переломами костей и повреждениями суставов Зат алмасу ауруларын тағаммен емдеу және емдік дене шынықтыру
Зат алмасу ауруларын тағаммен емдеу және емдік дене шынықтыру Долікарська допомога
Долікарська допомога Парентералды жолмен берілетін вирусты аурулар (ЖИТС) және олардың алдын алу шаралары
Парентералды жолмен берілетін вирусты аурулар (ЖИТС) және олардың алдын алу шаралары Логопедическое заключение. Направления работы при различных речевых нарушениях
Логопедическое заключение. Направления работы при различных речевых нарушениях Заикание. Этиология
Заикание. Этиология Сердечно- лёгочно-церебральная реанимация. Внебольничная остановка кровообращения
Сердечно- лёгочно-церебральная реанимация. Внебольничная остановка кровообращения Правила безопасного сексуального поведения
Правила безопасного сексуального поведения Медицинская генетика
Медицинская генетика Эпидемиология сахарного диабета (в РФ, субъектах РФ и других странах)
Эпидемиология сахарного диабета (в РФ, субъектах РФ и других странах) Бел, сегізкөз және құйымшақ жұлын нервтері
Бел, сегізкөз және құйымшақ жұлын нервтері Bronchitis in children
Bronchitis in children Тырыспаға қарсы және психотроптық дәрілік заттардың клиникалық фармакологиясы
Тырыспаға қарсы және психотроптық дәрілік заттардың клиникалық фармакологиясы Хронофармакология
Хронофармакология Заболевания сосудов. Нарушения венозного оттока
Заболевания сосудов. Нарушения венозного оттока Организация доклинических испытаний лекарственных препаратов
Организация доклинических испытаний лекарственных препаратов Қазіргі карпульды анестетиктер. Түрлері. Клиникалық фармокологиялық сипаттамасы. Стоматологиядағы жергілікті инъекциялық
Қазіргі карпульды анестетиктер. Түрлері. Клиникалық фармокологиялық сипаттамасы. Стоматологиядағы жергілікті инъекциялық Биологическая опасность. Понятие инфекционного и эпидемического процесса. Дезинфекция, дезинсекция. дератизация: понятия и виды
Биологическая опасность. Понятие инфекционного и эпидемического процесса. Дезинфекция, дезинсекция. дератизация: понятия и виды Анестезия в сердечно-сосудистой хирургии
Анестезия в сердечно-сосудистой хирургии Пиелонефрит у беременных
Пиелонефрит у беременных Переход к системе непрерывного медицинского (фармацевтического) образования
Переход к системе непрерывного медицинского (фармацевтического) образования Врачебные специальности. Послевузовсое образование врача-лечебника
Врачебные специальности. Послевузовсое образование врача-лечебника Психопатии. Патохарактерологическое развитие личности
Психопатии. Патохарактерологическое развитие личности Cтоп коронавирус
Cтоп коронавирус Панариций. Классификация и причины панариций
Панариций. Классификация и причины панариций Медицинада ДНҚ-диагностика әдісін қолдану
Медицинада ДНҚ-диагностика әдісін қолдану Фармакотерапия при гипертонической болезни
Фармакотерапия при гипертонической болезни