Содержание
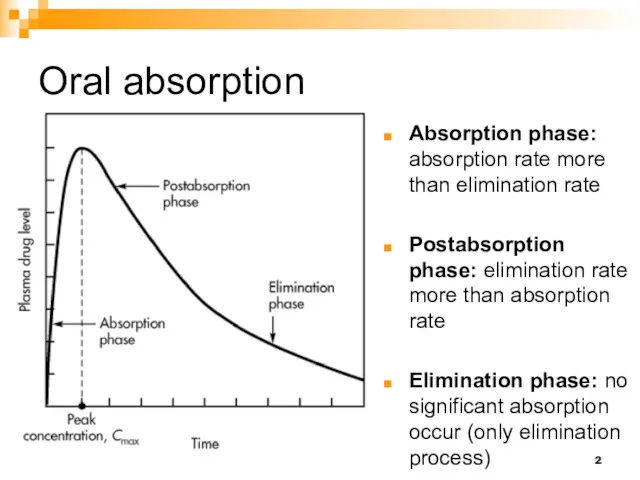
- 2. Oral absorption Absorption phase: absorption rate more than elimination rate Postabsorption phase: elimination rate more than
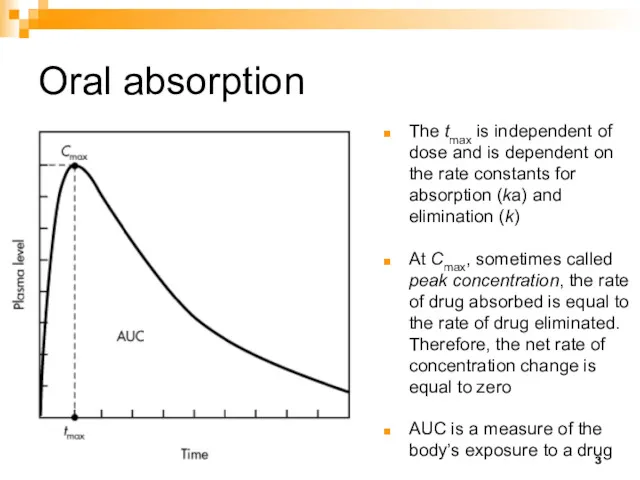
- 3. Oral absorption The tmax is independent of dose and is dependent on the rate constants for
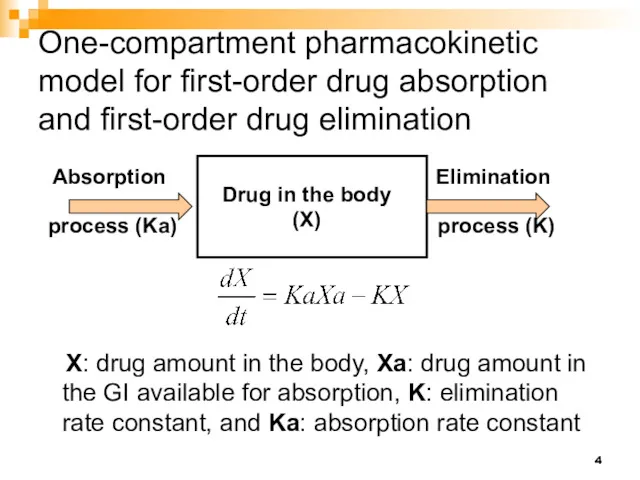
- 4. One-compartment pharmacokinetic model for first-order drug absorption and first-order drug elimination Drug in the body (X)
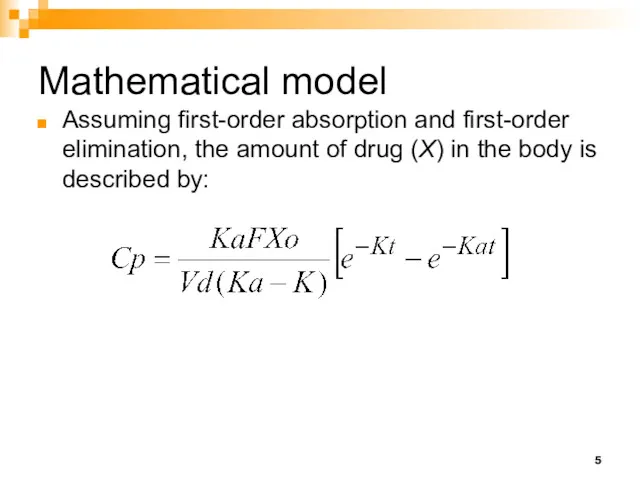
- 5. Mathematical model Assuming first-order absorption and first-order elimination, the amount of drug (X) in the body
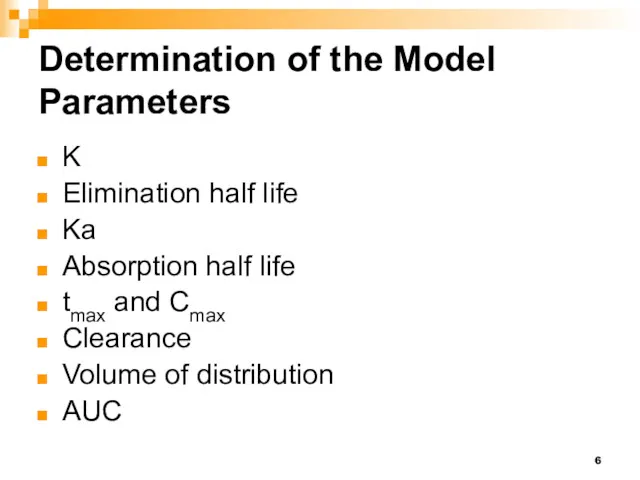
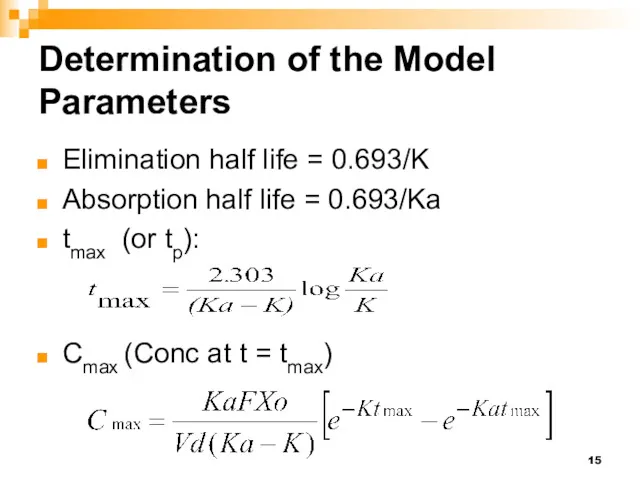
- 6. Determination of the Model Parameters K Elimination half life Ka Absorption half life tmax and Cmax
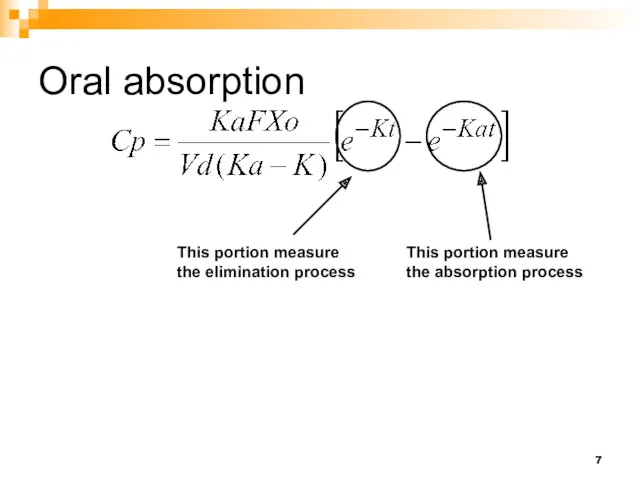
- 7. Oral absorption This portion measure the elimination process This portion measure the absorption process
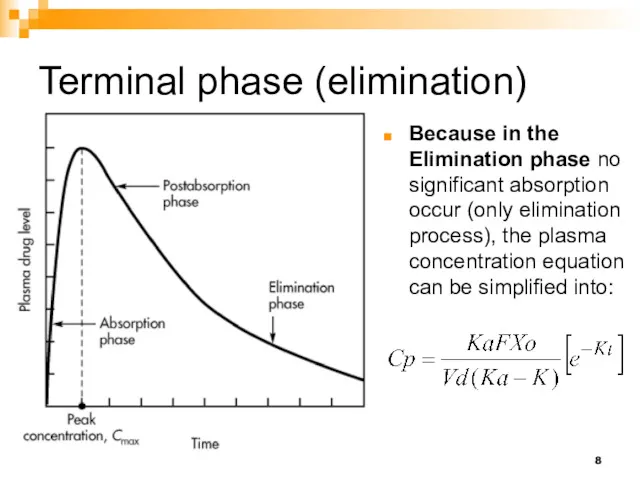
- 8. Terminal phase (elimination) Because in the Elimination phase no significant absorption occur (only elimination process), the
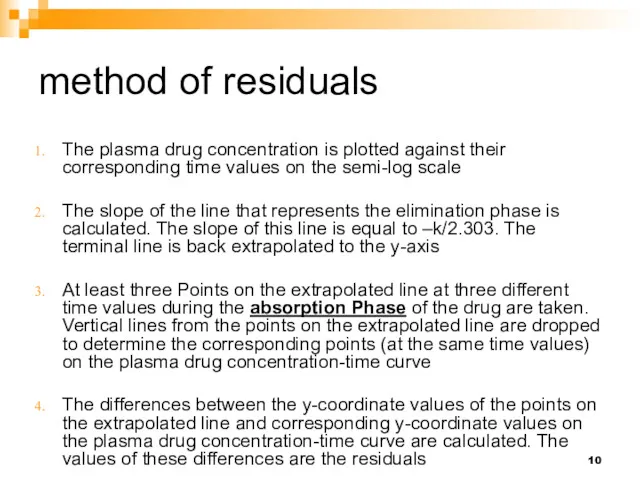
- 9. method of residuals The method of residuals is a graphical method used to determine the drug
- 10. method of residuals The plasma drug concentration is plotted against their corresponding time values on the
- 11. Method of residuals The values of the residuals are plotted versus their corresponding time values for
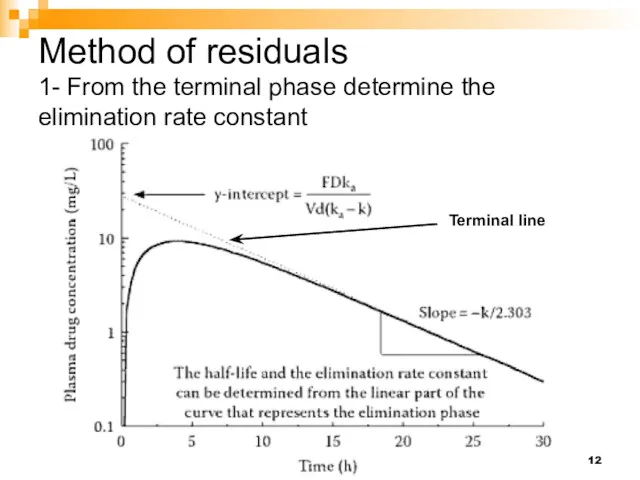
- 12. Method of residuals 1- From the terminal phase determine the elimination rate constant Terminal line
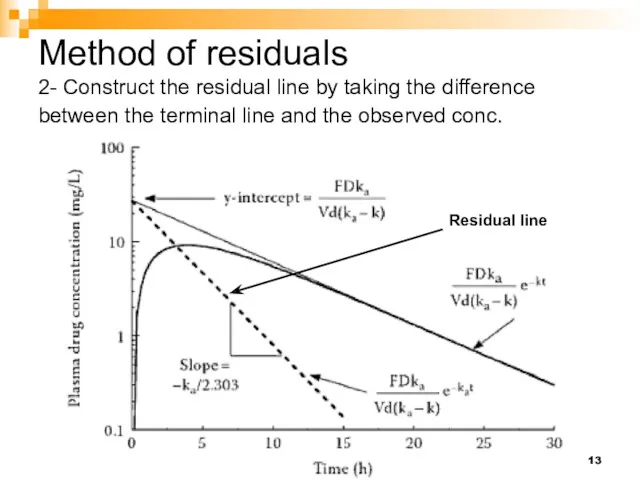
- 13. Method of residuals 2- Construct the residual line by taking the difference between the terminal line
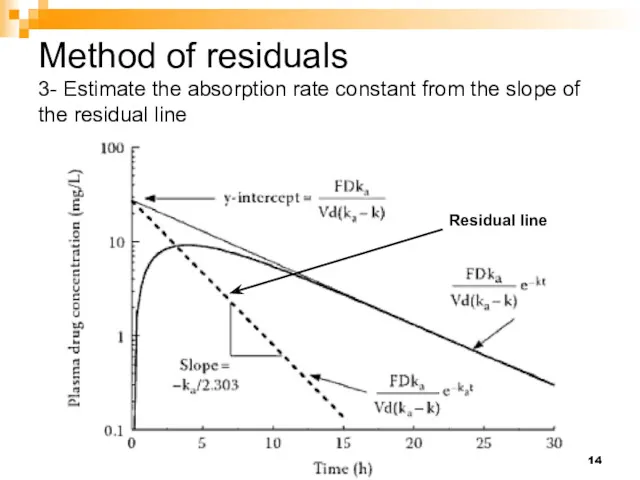
- 14. Method of residuals 3- Estimate the absorption rate constant from the slope of the residual line
- 15. Determination of the Model Parameters Elimination half life = 0.693/K Absorption half life = 0.693/Ka tmax
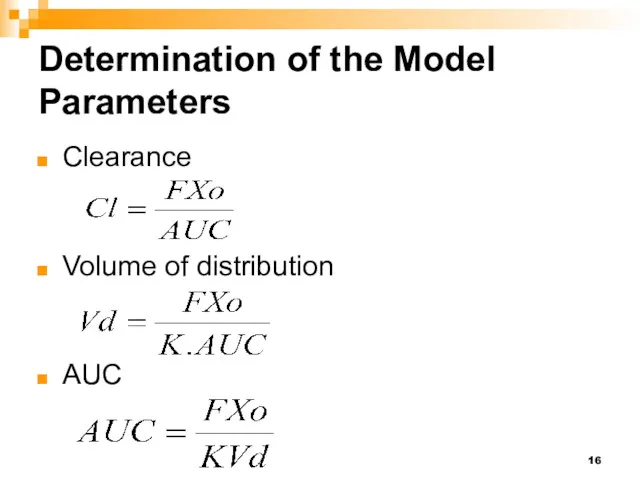
- 16. Determination of the Model Parameters Clearance Volume of distribution AUC
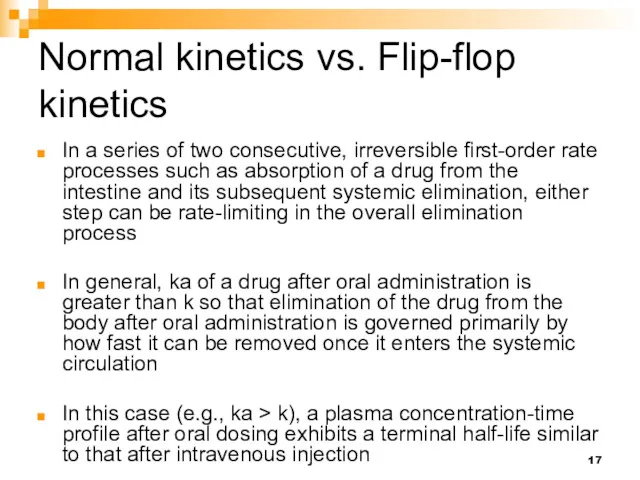
- 17. Normal kinetics vs. Flip-flop kinetics In a series of two consecutive, irreversible first-order rate processes such
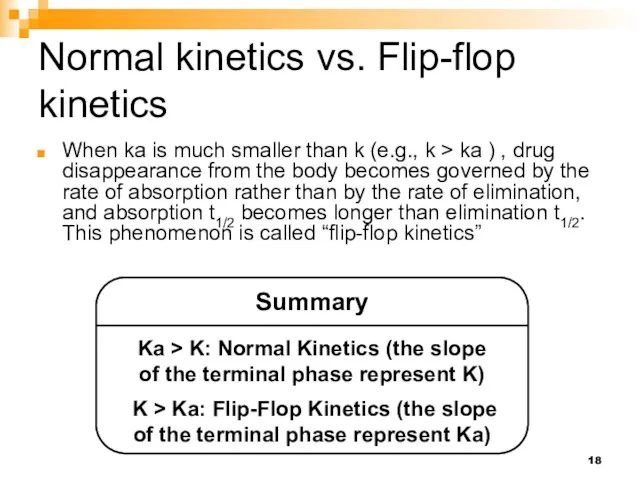
- 18. Normal kinetics vs. Flip-flop kinetics When ka is much smaller than k (e.g., k > ka
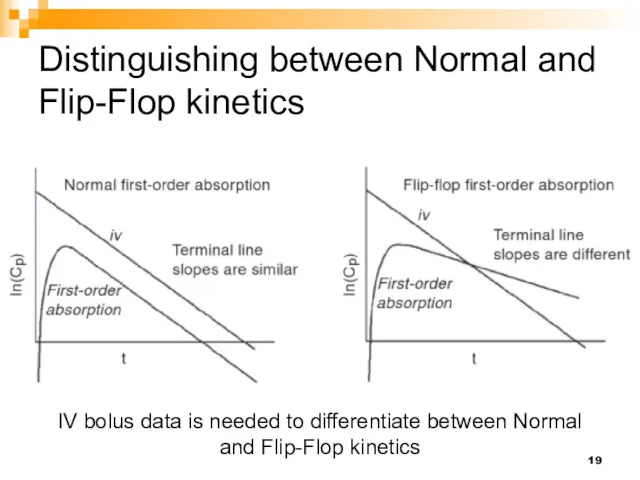
- 19. Distinguishing between Normal and Flip-Flop kinetics IV bolus data is needed to differentiate between Normal and
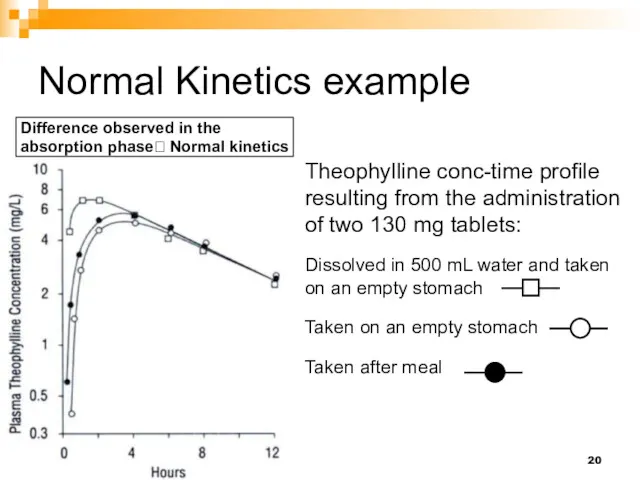
- 20. Normal Kinetics example Theophylline conc-time profile resulting from the administration of two 130 mg tablets: Dissolved
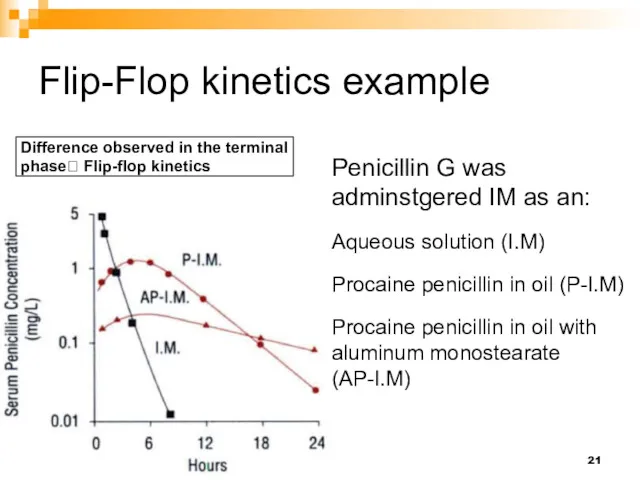
- 21. Flip-Flop kinetics example Penicillin G was adminstgered IM as an: Aqueous solution (I.M) Procaine penicillin in
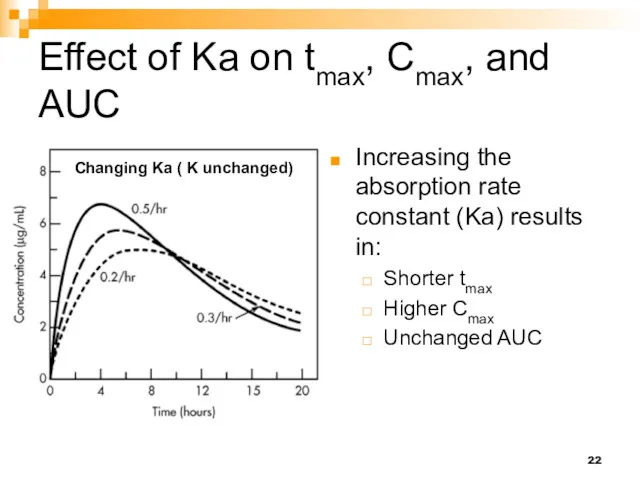
- 22. Effect of Ka on tmax, Cmax, and AUC Increasing the absorption rate constant (Ka) results in:
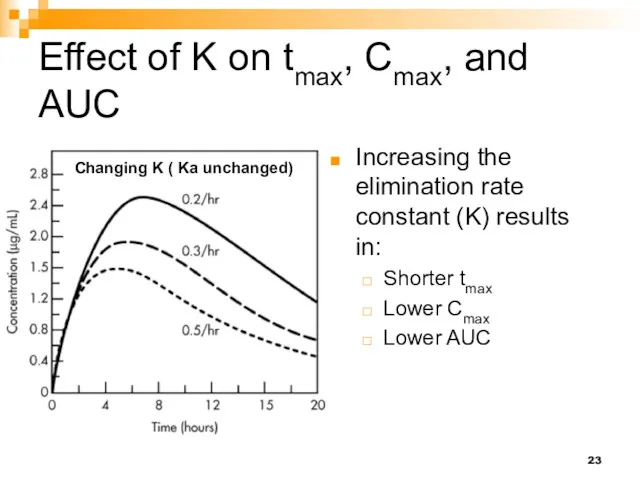
- 23. Effect of K on tmax, Cmax, and AUC Increasing the elimination rate constant (K) results in:
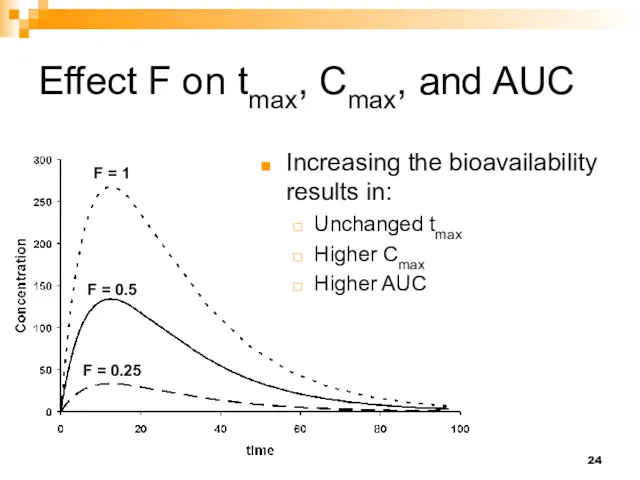
- 24. Effect F on tmax, Cmax, and AUC F = 1 F = 0.5 F = 0.25
- 25. Bioavailability Systemic absorption is often incomplete when given extravascularly Knowing the extent of absorption (bioavailability) helps
- 26. Bioavailability To determine clearance, a drug must be given intravascularly, as only then is the amount
- 27. Bioavailability If the IV and oral doses were equal, F can be calculated according to:
- 28. Example 1 A 500-mg dose of the sulfonamide sulfamethoxazole is administered as an oral tablet to
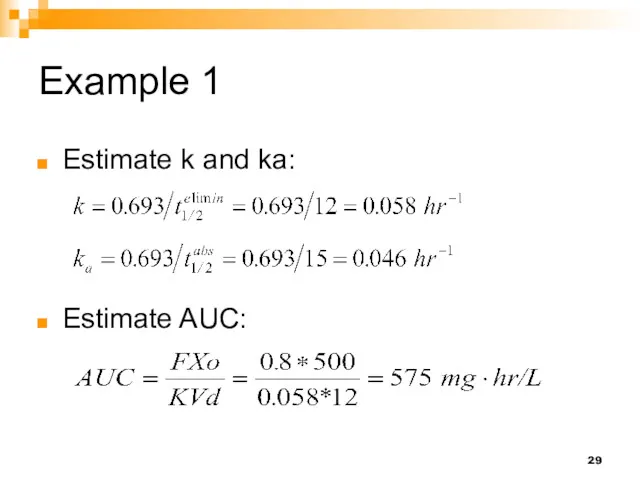
- 29. Example 1 Estimate k and ka: Estimate AUC:
- 30. Example 1 Estimate tmax:
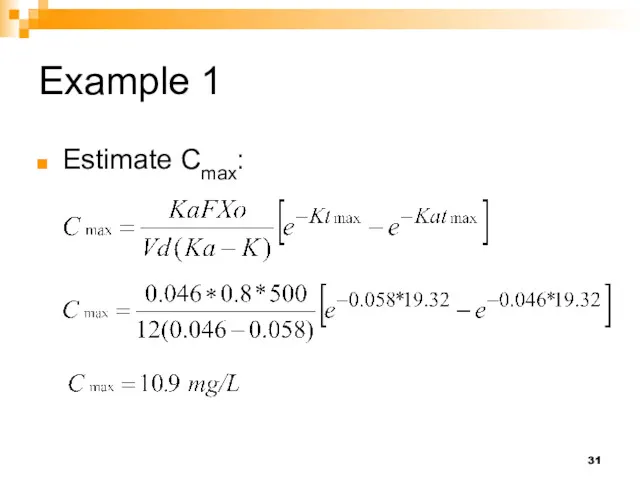
- 31. Example 1 Estimate Cmax:
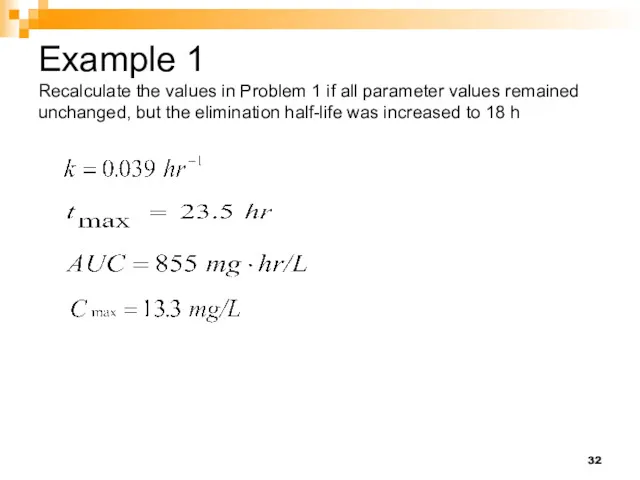
- 32. Example 1 Recalculate the values in Problem 1 if all parameter values remained unchanged, but the
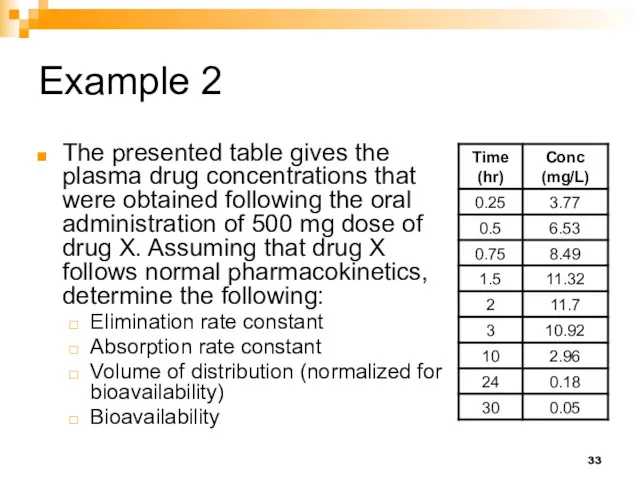
- 33. Example 2 The presented table gives the plasma drug concentrations that were obtained following the oral
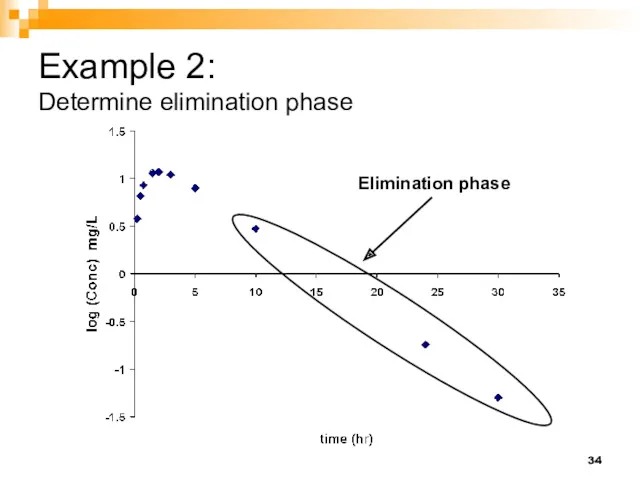
- 34. Example 2: Determine elimination phase Elimination phase
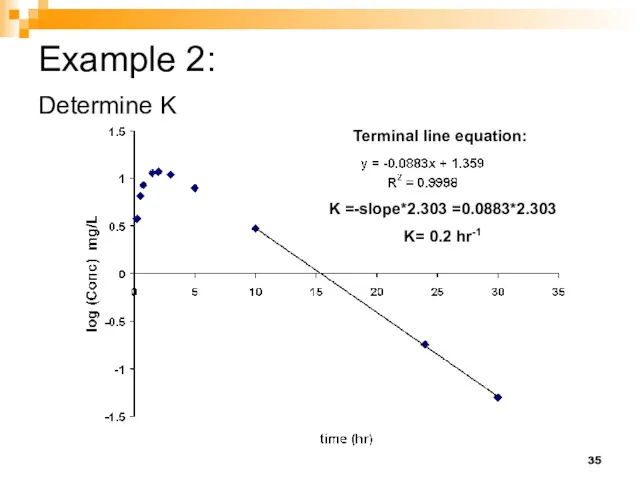
- 35. Example 2: Determine K Terminal line equation: K =-slope*2.303 =0.0883*2.303 K= 0.2 hr-1
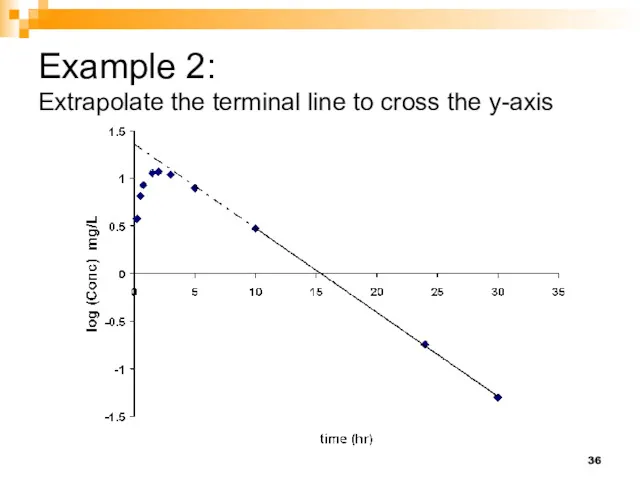
- 36. Example 2: Extrapolate the terminal line to cross the y-axis
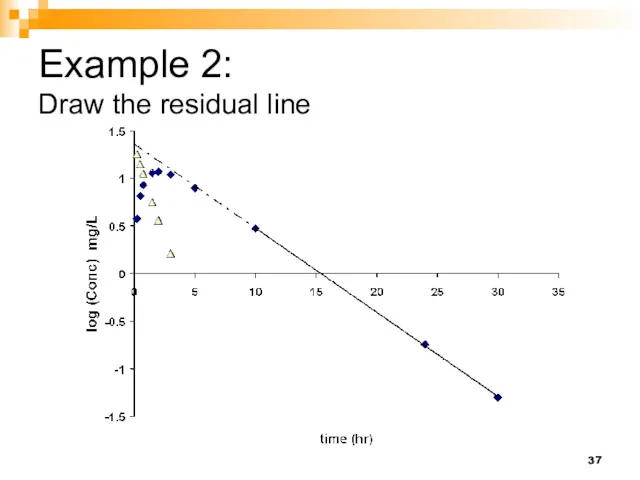
- 37. Example 2: Draw the residual line
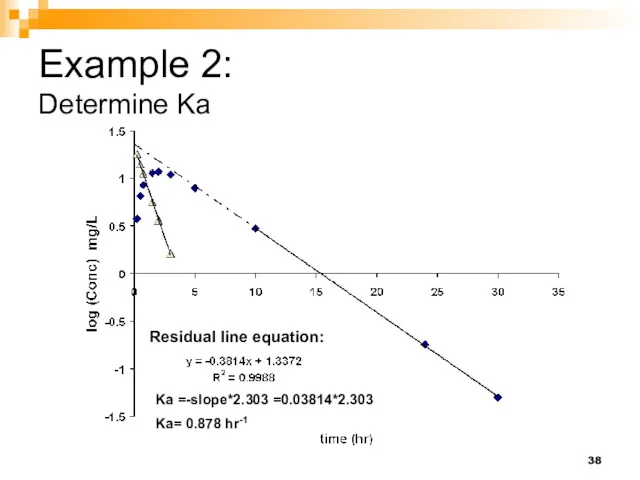
- 38. Example 2: Determine Ka Residual line equation: Ka =-slope*2.303 =0.03814*2.303 Ka= 0.878 hr-1
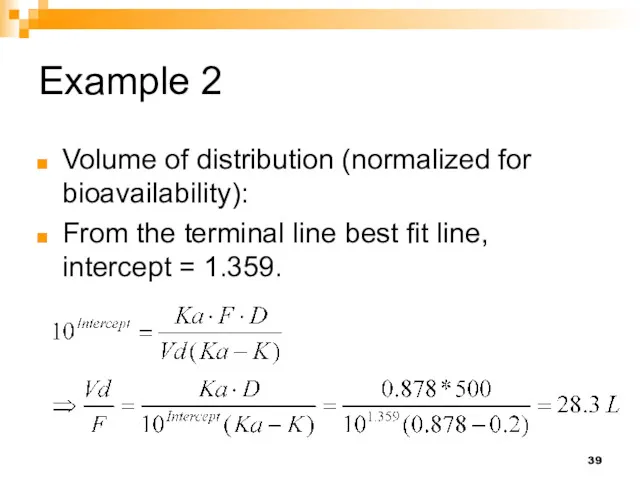
- 39. Example 2 Volume of distribution (normalized for bioavailability): From the terminal line best fit line, intercept
- 40. Example 3 A patient received a single dose of 500 mg erythromycin in the form of
- 42. Скачать презентацию






 Герпесвирусные инфекции у детей: современные аспекты диагностики и лечения
Герпесвирусные инфекции у детей: современные аспекты диагностики и лечения Дистрофия. Морфологическая сущность и морфогенез дистрофий
Дистрофия. Морфологическая сущность и морфогенез дистрофий Пищевая аддикция
Пищевая аддикция Патология детей раннего возраста. Рахит
Патология детей раннего возраста. Рахит Хронический гастрит. Язвенная болезнь
Хронический гастрит. Язвенная болезнь Основы клинической физиологии сердца
Основы клинической физиологии сердца Туберкулез. Туберкулездің алдын алу спецификалық және спецификалық емес түрі
Туберкулез. Туберкулездің алдын алу спецификалық және спецификалық емес түрі Рак желудка
Рак желудка Иммунный статус и его оценка
Иммунный статус и его оценка Первая медицинская помощь при ДТП
Первая медицинская помощь при ДТП Наследственные нарушения развития тканей зубов
Наследственные нарушения развития тканей зубов Гипноз сегодня. Мифы и реальность
Гипноз сегодня. Мифы и реальность Подготовка к летней оздоровительной кампании в г. Архангельске в 2018 году
Подготовка к летней оздоровительной кампании в г. Архангельске в 2018 году Особенности кровообращения плода и детей раннего возраста
Особенности кровообращения плода и детей раннего возраста Методы патопсихологии
Методы патопсихологии Амбулатория жағдайында іш қуысының ауырсынуы кезіндегі рационалды дифференциалды диагностика алгоритмі
Амбулатория жағдайында іш қуысының ауырсынуы кезіндегі рационалды дифференциалды диагностика алгоритмі Антимикробные химиотерапевтические препараты. Механизмы действия. Осложнения антимикробной терапии
Антимикробные химиотерапевтические препараты. Механизмы действия. Осложнения антимикробной терапии Политравма. Этиология и эпидемиология политравмы
Политравма. Этиология и эпидемиология политравмы Мерез ауруы кезіндегі ауыз қуысының шырышты қабатындағы көріністер
Мерез ауруы кезіндегі ауыз қуысының шырышты қабатындағы көріністер Нижний этаж брюшной полости
Нижний этаж брюшной полости Біліктік қаңқа. Типті омыртқаның құрылысы
Біліктік қаңқа. Типті омыртқаның құрылысы Острый одонтогенный, гематогенный, хронический остеомиелит
Острый одонтогенный, гематогенный, хронический остеомиелит Клиника, диагностика, дифференциальная диагностика среднего, быстрого и медленно прогрессирующего глубокого кариеса
Клиника, диагностика, дифференциальная диагностика среднего, быстрого и медленно прогрессирующего глубокого кариеса Теория по стоматологии. Гигиена. Отбеливание
Теория по стоматологии. Гигиена. Отбеливание Диагностика. Фотографии лица до лечения
Диагностика. Фотографии лица до лечения Иммунитет. Термины. Виды иммунитета. ВИЧ и СПИД
Иммунитет. Термины. Виды иммунитета. ВИЧ и СПИД Заболевания надпочечников. Синдром Иценко-Кушинга. Болезнь Аддисона
Заболевания надпочечников. Синдром Иценко-Кушинга. Болезнь Аддисона Лекарственные средства, влияющие на афферентную нервную систему
Лекарственные средства, влияющие на афферентную нервную систему