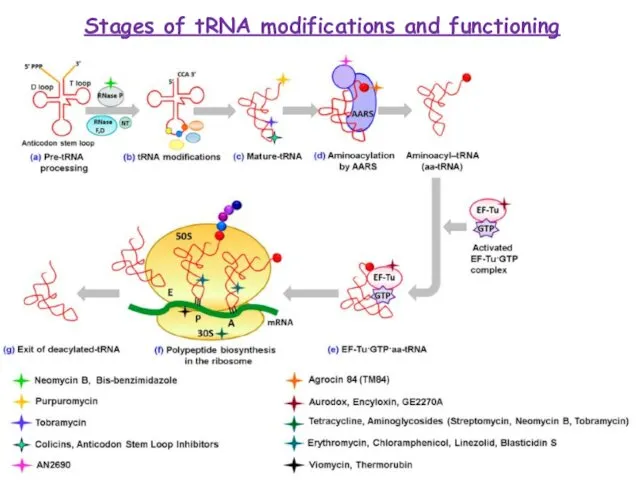
Слайд 15

REFERENCE
Chopra Sh., Reader J., tRNAs as Antibiotic Targets. Int. J.
Mol. Sci. 2015, 16, 321-349
Watson J D, Baker T A , Bell S P, Gann A, Levine M, Losick R. Molecular Biology of the Gene. 5th edition. Pearson education 2004.
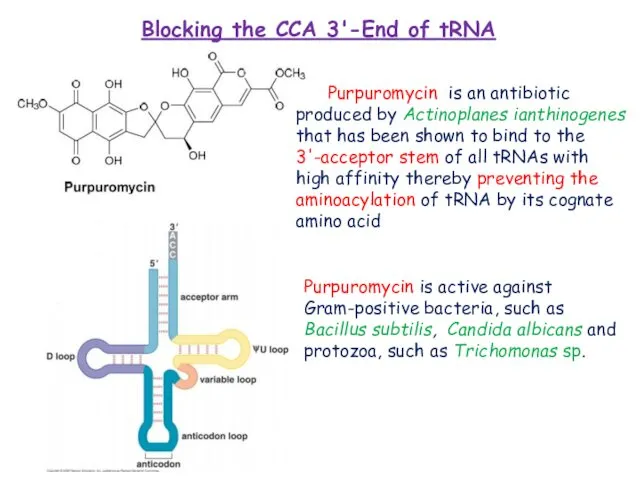
Kirillov, S.; Vitali, L.A.; Goldstein, B.P.; Monti, F.; Semenkov, Y.; Makhno, V.; Ripa, S.; Pon, C.L.; Gualerzi, C.O. Purpuromycin: An antibiotic inhibiting tRNA aminoacylation. RNA 1997, 3, 905–913.
Landini, P.; Corti, E.; Goldstein, B.P.; Denaro, M. Mechanism of action of purpuromycin. Biochem. J. 1992, 284, 935.
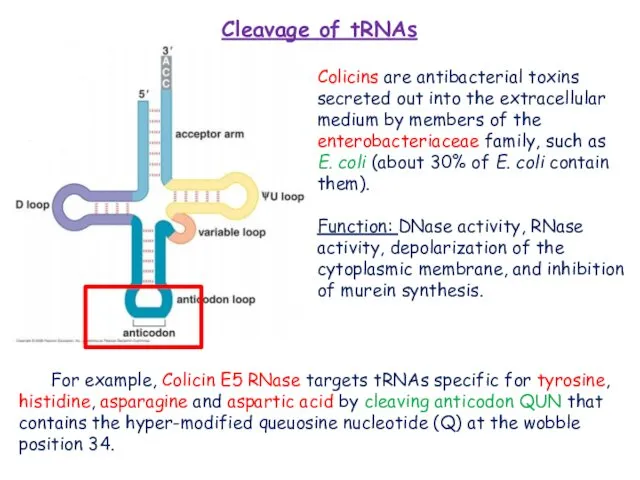
Tomita, K.; Ogawa, T.; Uozumi, T.; Watanabe, K.; Masaki, H. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc. Natl. Acad. Sci. USA 2000, 97, 8278–8283.
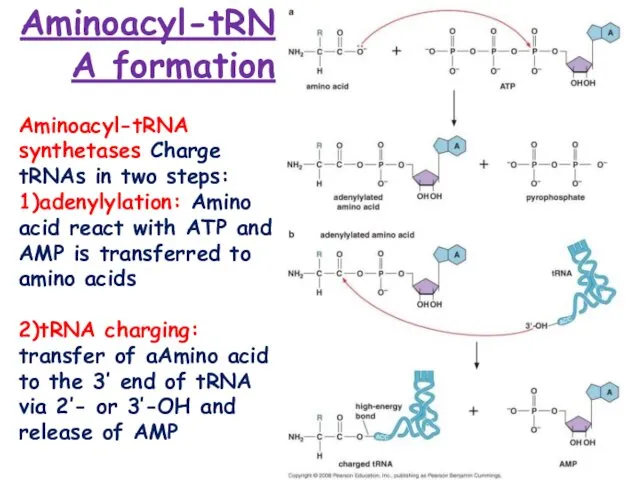
Chen, J.F.; Guo, N.N.; Li, T.; Wang, E.D.; Wang, Y.L. CP1 domain in Escherichia coli leucyl-tRNA synthetase is crucial for its editing function. Biochemistry 2000, 39, 6726 6731.
Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 2007, 316, 1759–1761.
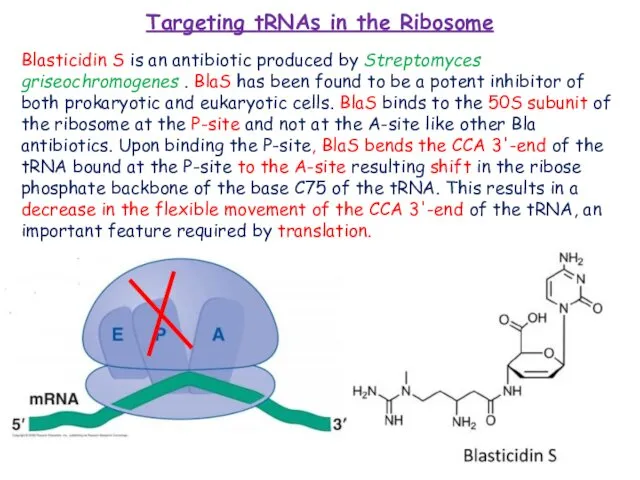



 IT – Скорая. Организация по ремонту компьютеров
IT – Скорая. Организация по ремонту компьютеров Основной курс: урок 27
Основной курс: урок 27 Взаимоотношения внутри популяции
Взаимоотношения внутри популяции Личность. Развитие личности: самосознание, самооценка
Личность. Развитие личности: самосознание, самооценка Туристские возможности родного края
Туристские возможности родного края Организация высшего образования в РФ. Лицензирование, аккредитация и аттестация вузов. Государственные образовательные стандарты
Организация высшего образования в РФ. Лицензирование, аккредитация и аттестация вузов. Государственные образовательные стандарты Безопасность на дороге
Безопасность на дороге Психология человека
Психология человека Керування проектами
Керування проектами Внутреннее убранство русской избы
Внутреннее убранство русской избы Механизация поверхностной обработки почвы при возделывании озимой пшеницы
Механизация поверхностной обработки почвы при возделывании озимой пшеницы Дизайн для реального мира. Виктор Папанек (1923-1980)
Дизайн для реального мира. Виктор Папанек (1923-1980) Презентация к уроку Птичьи разговоры
Презентация к уроку Птичьи разговоры Религиозный этикет в праздниках. Никах
Религиозный этикет в праздниках. Никах Дидактические игры по развитию речи
Дидактические игры по развитию речи Подобные треугольники
Подобные треугольники Октябрьская революция 1917 года в России
Октябрьская революция 1917 года в России Единицы объёма. Решение задач на нахождение объёма
Единицы объёма. Решение задач на нахождение объёма Самопрезентация
Самопрезентация Значение разминки в физической культуре
Значение разминки в физической культуре Организация прогулок для детей раннего возраста в условиях Крайнего Севера
Организация прогулок для детей раннего возраста в условиях Крайнего Севера Алжи́рская Наро́дная Демократи́ческая Республика
Алжи́рская Наро́дная Демократи́ческая Республика презентация Великой Победе посвящается
презентация Великой Победе посвящается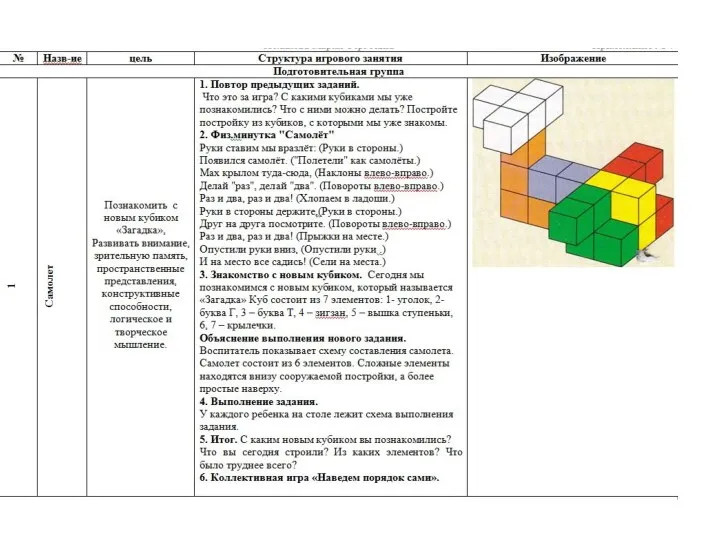 Перспективное планирование работы с Логическими кубиками для всех Б.П.Никтина подготовительная группа
Перспективное планирование работы с Логическими кубиками для всех Б.П.Никтина подготовительная группа Место СМИ во взаимодействии власти и общества
Место СМИ во взаимодействии власти и общества Игра по ПДД На улицах и дорогах
Игра по ПДД На улицах и дорогах Авиационный транспорт Украины
Авиационный транспорт Украины Москва и ее жители в ХVI веке
Москва и ее жители в ХVI веке