Содержание
- 2. Content Introduction Classification Structure Mechanism of action 4.1 Activation and regulation
- 3. ntroduction Enzyme-linked receptors (catalytic receptos) are a second major type of cell-surface receptor. They were recognized
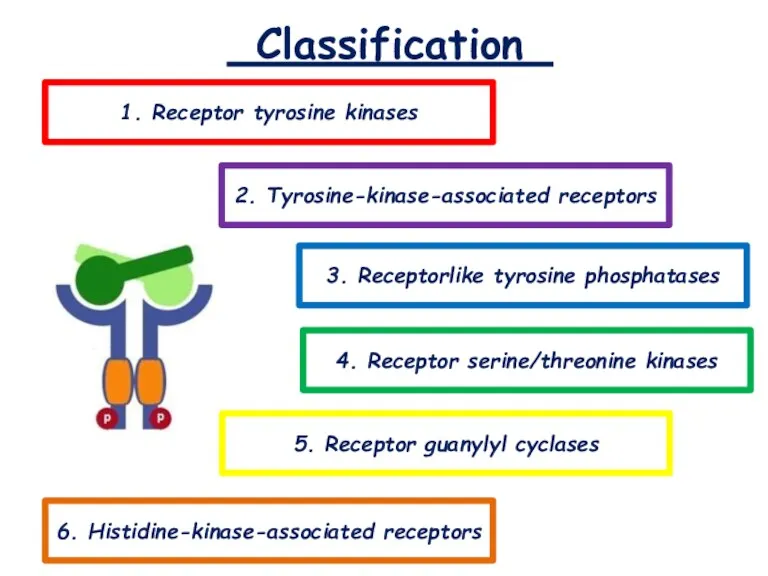
- 4. Classification 1. Receptor tyrosine kinases 2. Tyrosine-kinase-associated receptors 3. Receptorlike tyrosine phosphatases 5. Receptor guanylyl cyclases
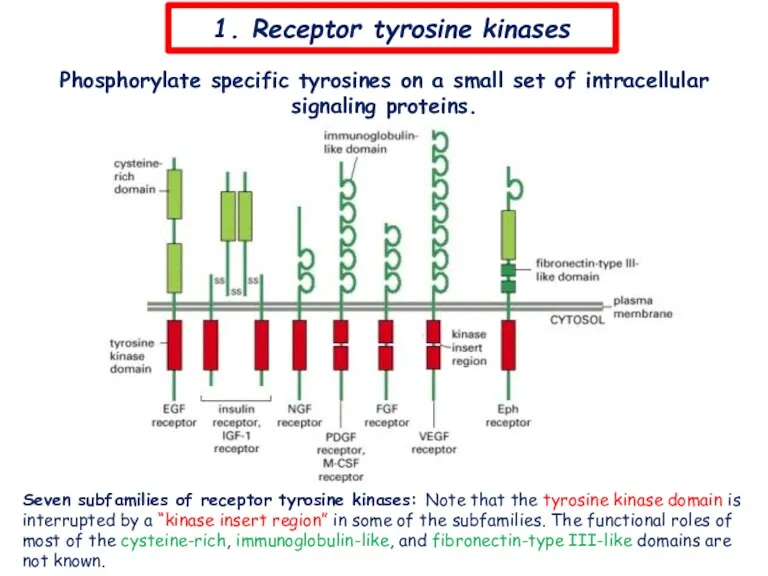
- 5. 1. Receptor tyrosine kinases Seven subfamilies of receptor tyrosine kinases: Note that the tyrosine kinase domain
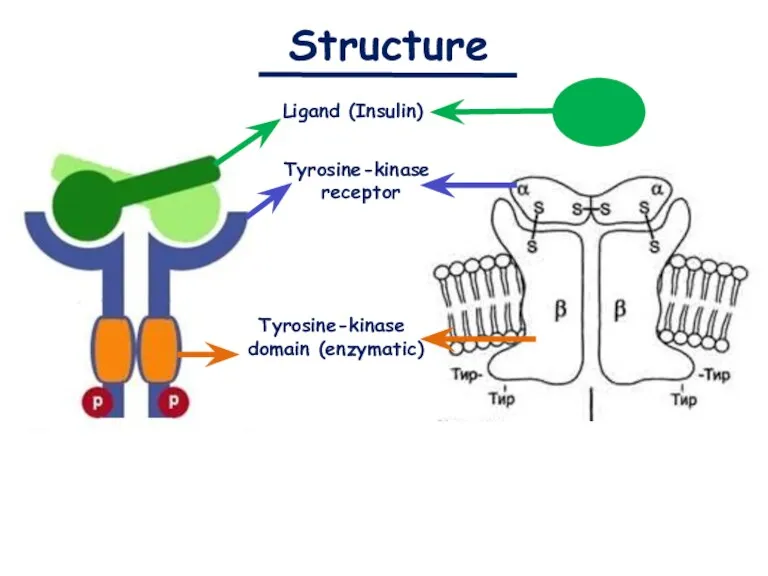
- 7. Structure Ligand (Insulin) Tyrosine-kinase receptor Tyrosine-kinase domain (enzymatic)
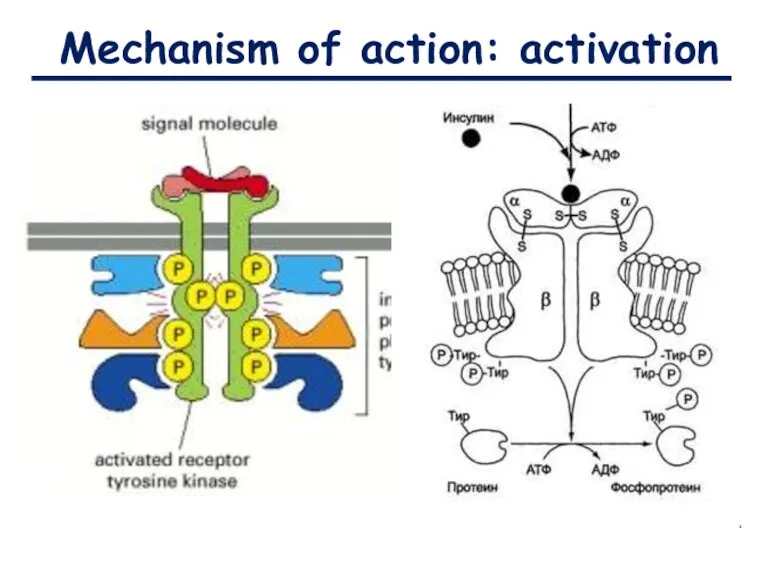
- 8. Mechanism of action: activation
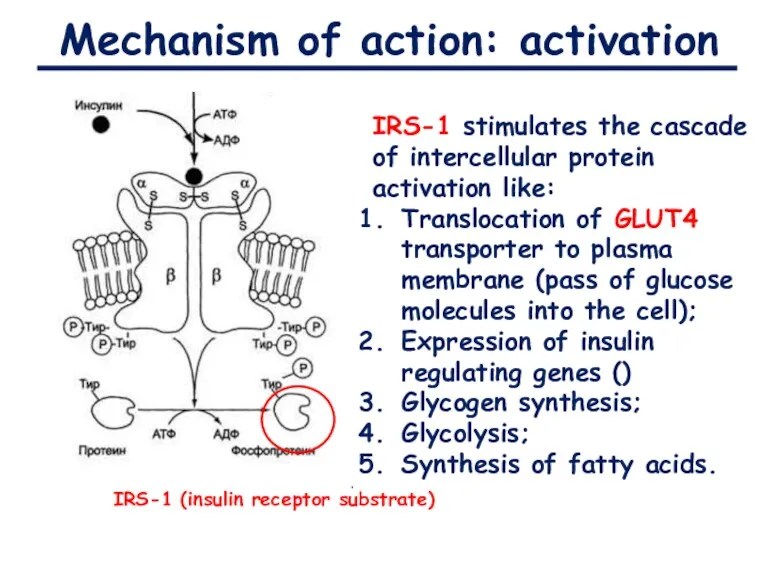
- 9. Mechanism of action: activation IRS-1 (insulin receptor substrate) IRS-1 stimulates the cascade of intercellular protein activation
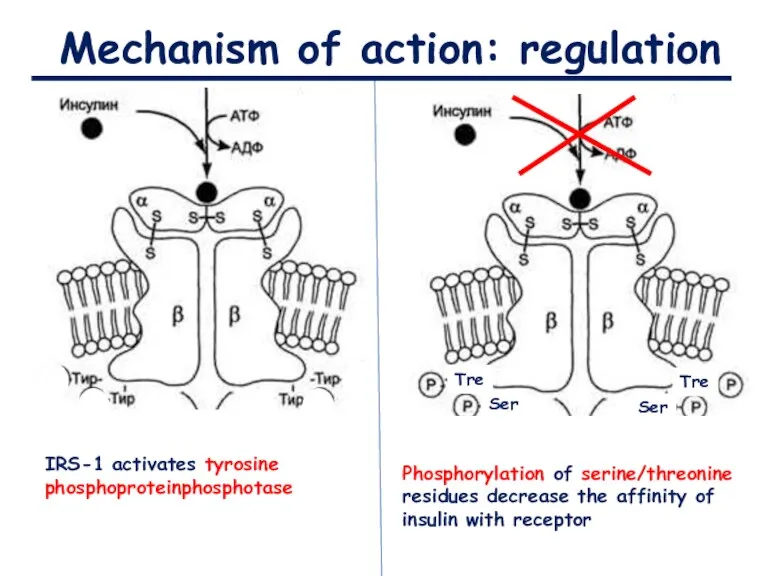
- 10. Mechanism of action: regulation IRS-1 activates tyrosine phosphoproteinphosphotase Phosphorylation of serine/threonine residues decrease the affinity of
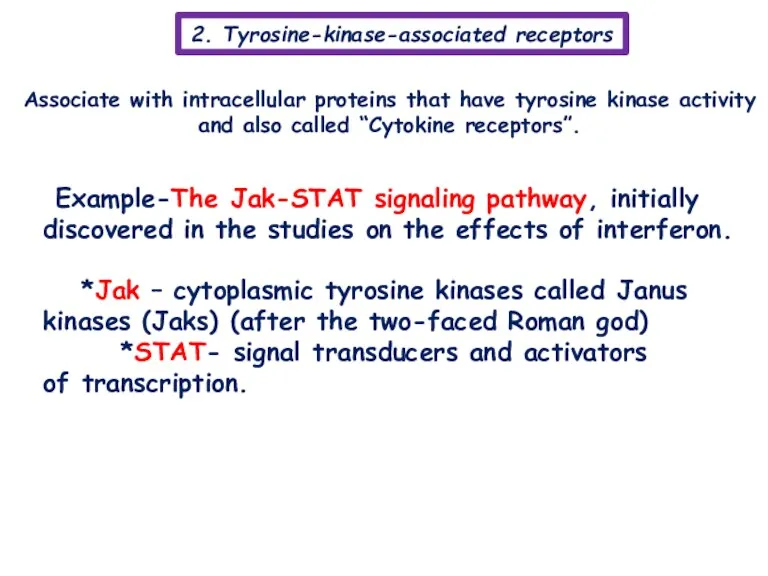
- 11. 2. Tyrosine-kinase-associated receptors Associate with intracellular proteins that have tyrosine kinase activity and also called “Cytokine
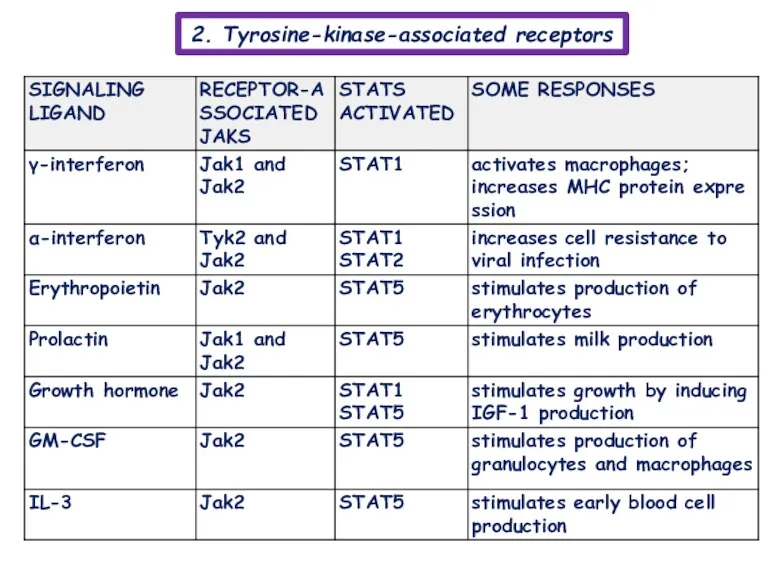
- 12. 2. Tyrosine-kinase-associated receptors
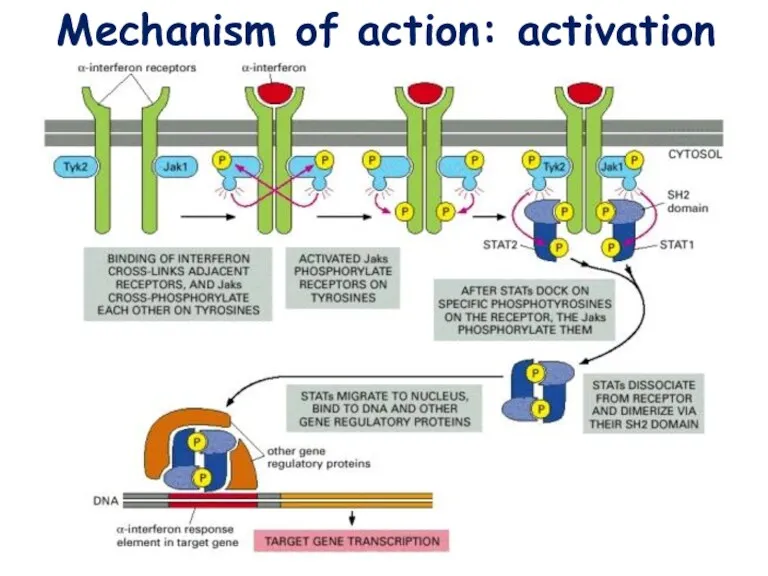
- 13. Mechanism of action: activation
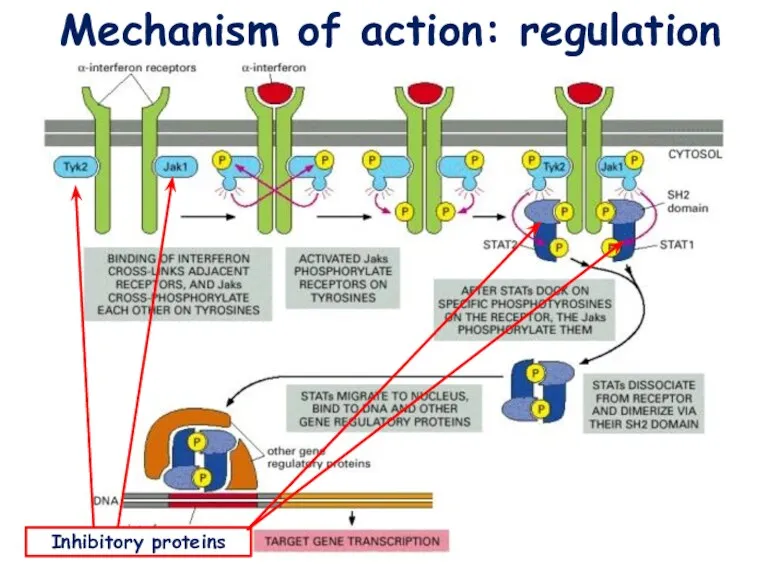
- 14. Mechanism of action: regulation Inhibitory proteins
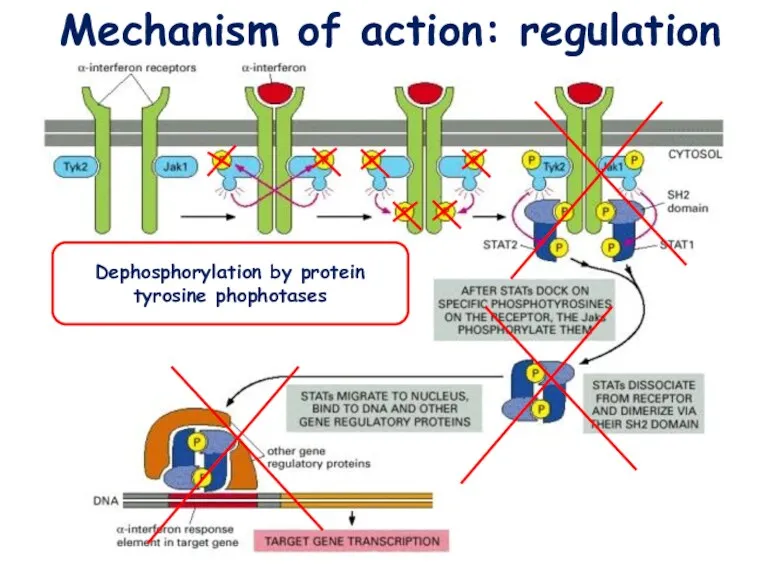
- 15. Mechanism of action: regulation Dephosphorylation by protein tyrosine phophotases
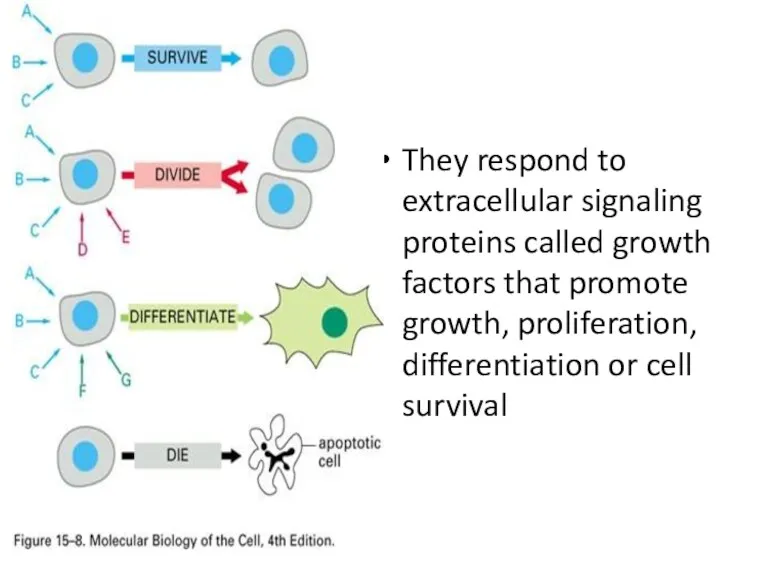
- 16. They respond to extracellular signaling proteins called growth factors that promote growth, proliferation, differentiation or cell
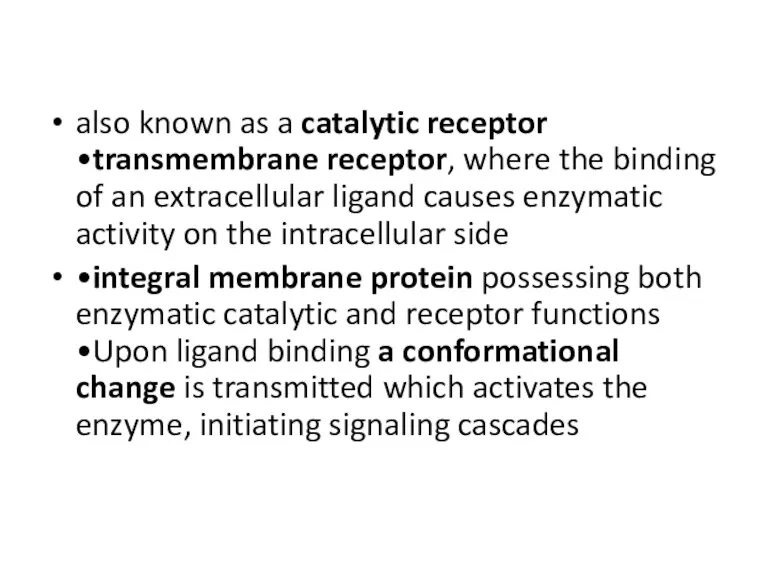
- 17. also known as a catalytic receptor •transmembrane receptor, where the binding of an extracellular ligand causes
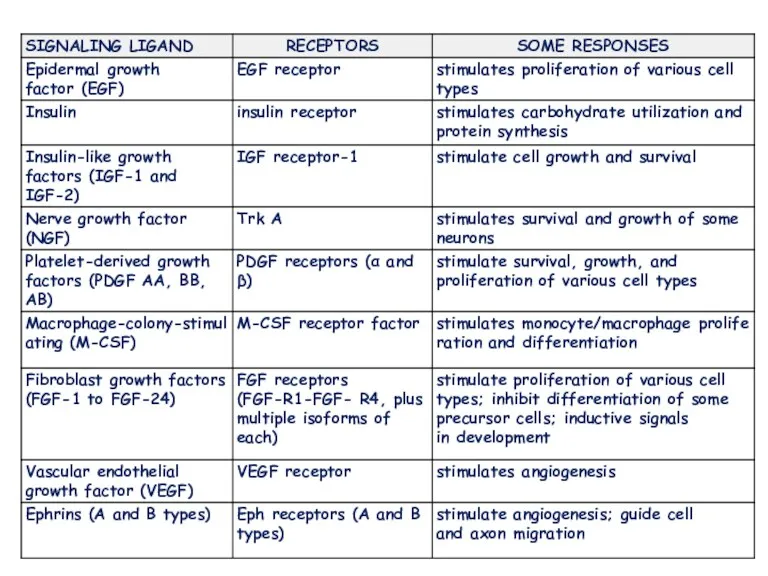
- 18. Physiology and diseases involved in growth, proliferation, differentiation, or survival Because of this, their ligands are
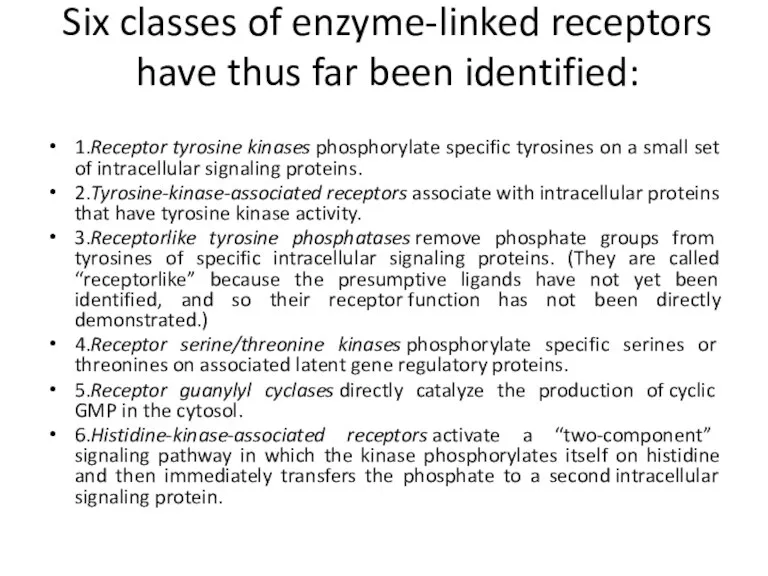
- 19. Six classes of enzyme-linked receptors have thus far been identified: 1.Receptor tyrosine kinases phosphorylate specific tyrosines
- 20. In enzymology, a receptor protein serine/threonine kinase (EC 2.7.11.30) is an enzyme that catalyzes the chemical
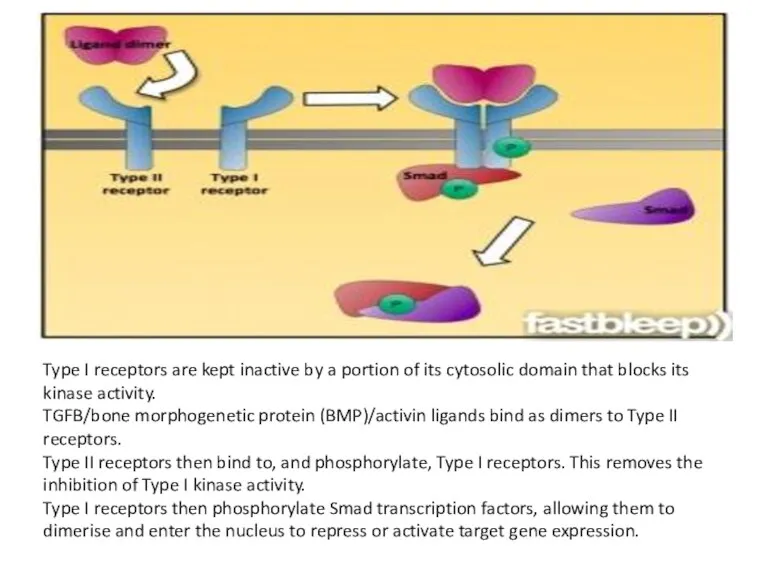
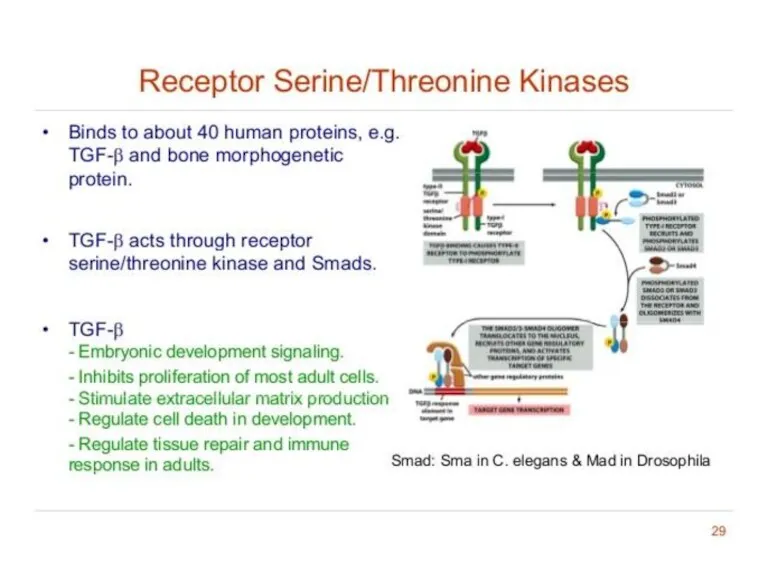
- 21. Receptor serine/threonine kinases phosphorylate specific Serine/ Threonine There are two types of serine/threonine kinase receptors, both
- 22. Type I receptors are kept inactive by a portion of its cytosolic domain that blocks its
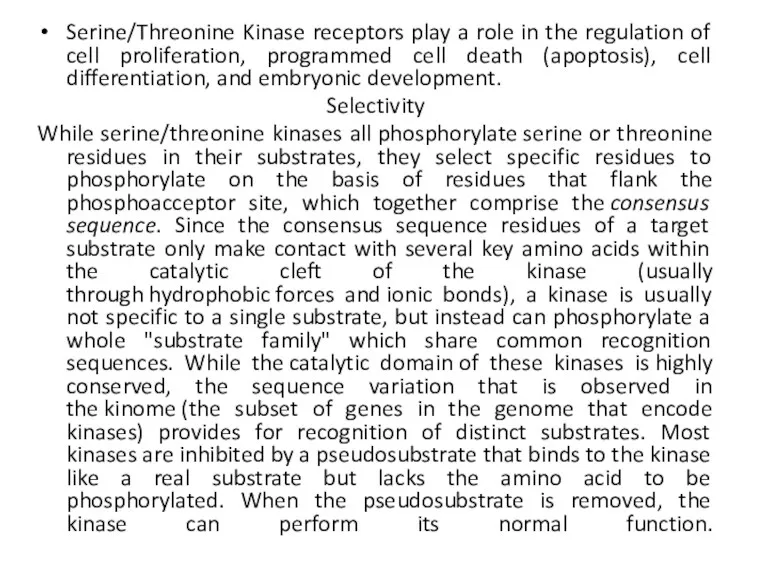
- 23. Serine/Threonine Kinase receptors play a role in the regulation of cell proliferation, programmed cell death (apoptosis),
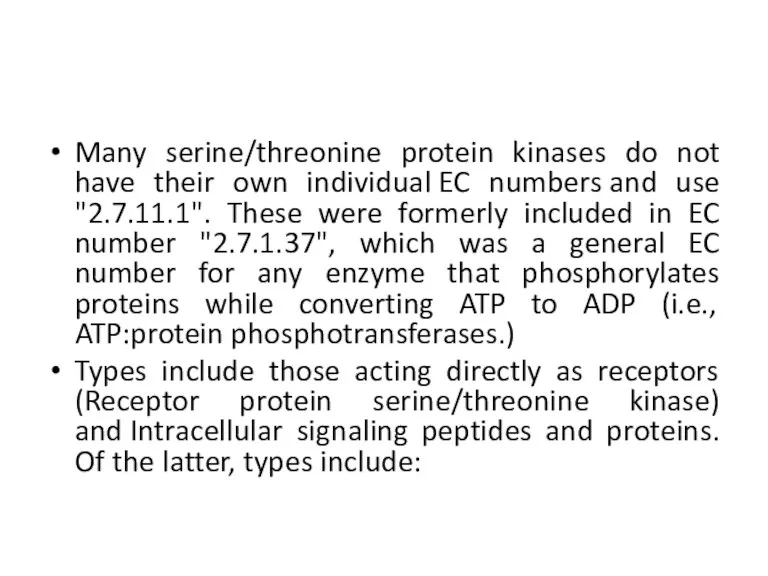
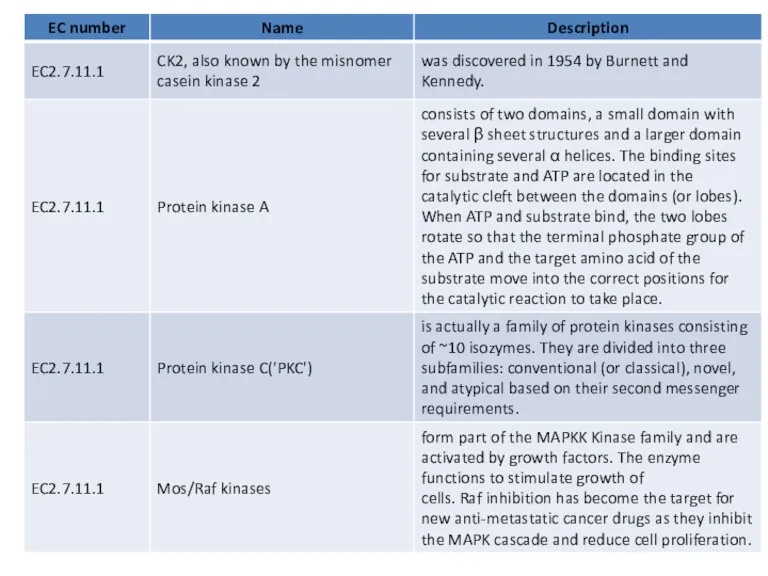
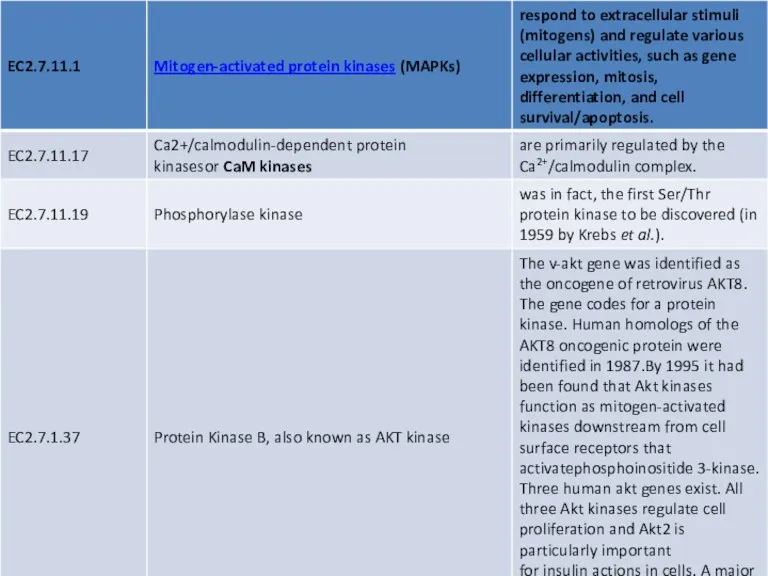
- 24. Many serine/threonine protein kinases do not have their own individual EC numbers and use "2.7.11.1". These
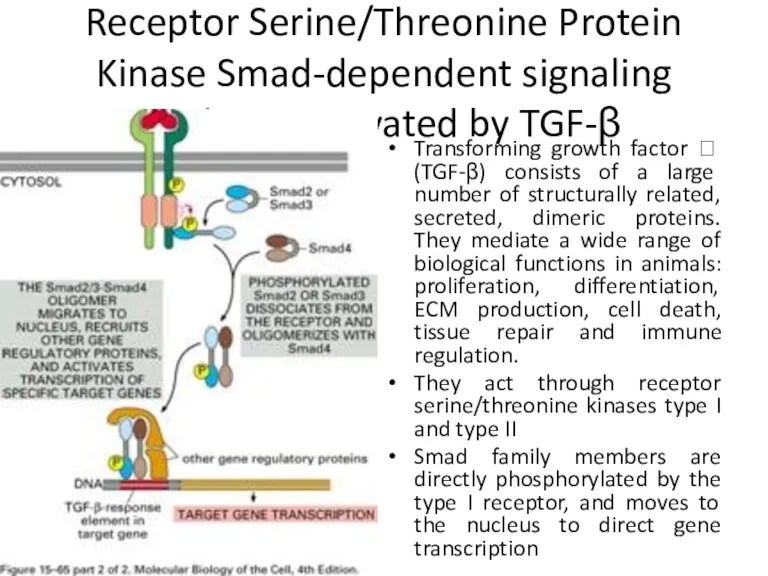
- 28. Receptor Serine/Threonine Protein Kinase Smad-dependent signaling pathway activated by TGF-β Transforming growth factor (TGF-β) consists
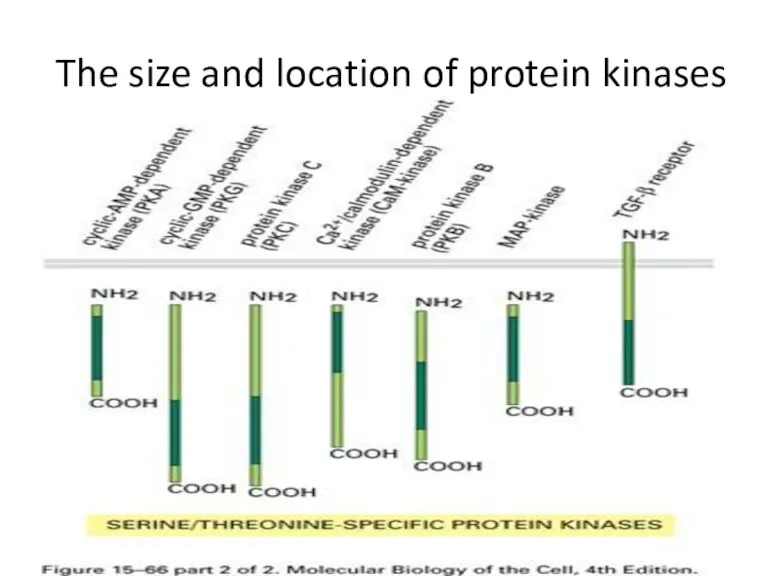
- 29. The size and location of protein kinases
- 30. Receptor like tyrosine phosphatases Receptor like tyrosine phosphatases remove phosphate groups from tyrosines of specific intracellular
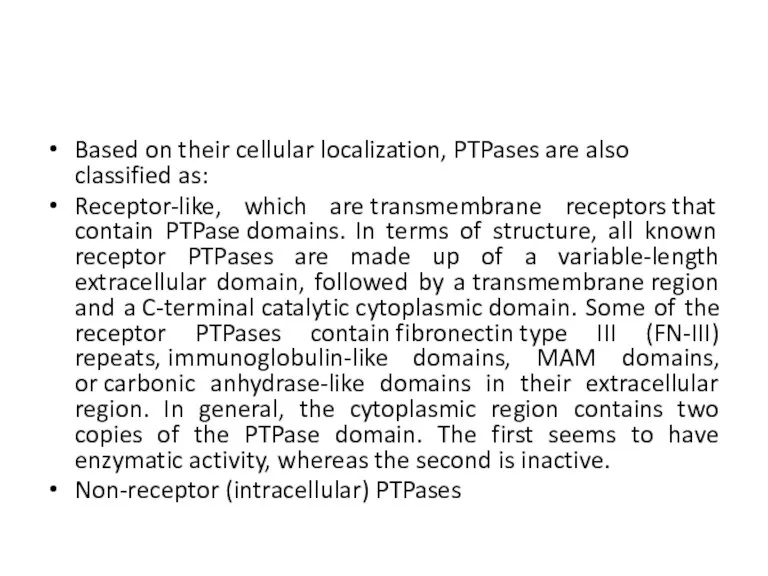
- 31. Based on their cellular localization, PTPases are also classified as: Receptor-like, which are transmembrane receptors that
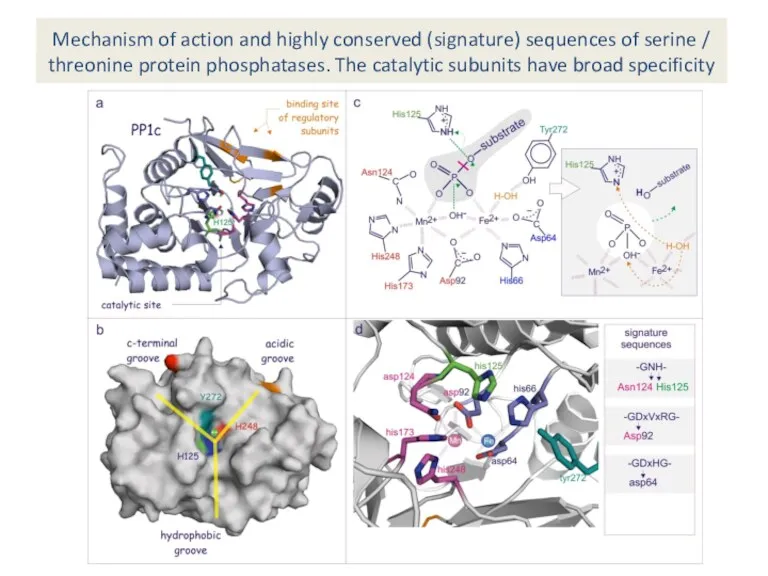
- 32. Mechanism of action and highly conserved (signature) sequences of serine / threonine protein phosphatases. The catalytic
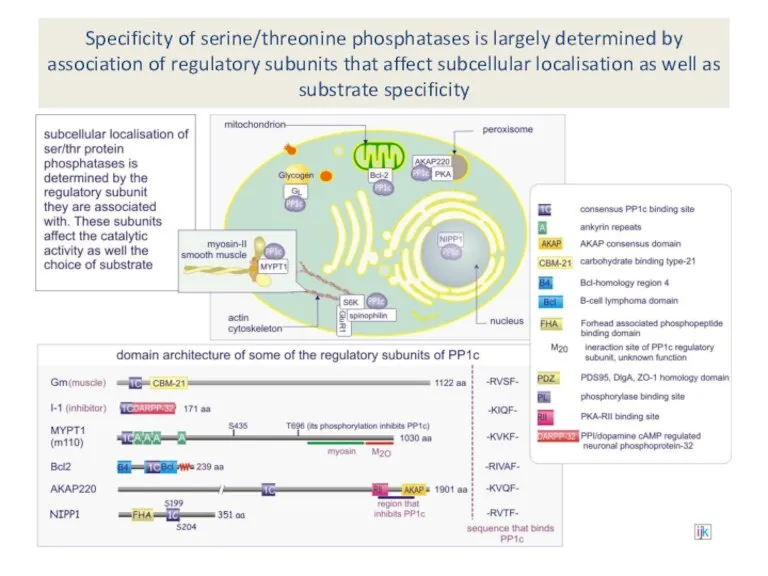
- 33. Specificity of serine/threonine phosphatases is largely determined by association of regulatory subunits that affect subcellular localisation
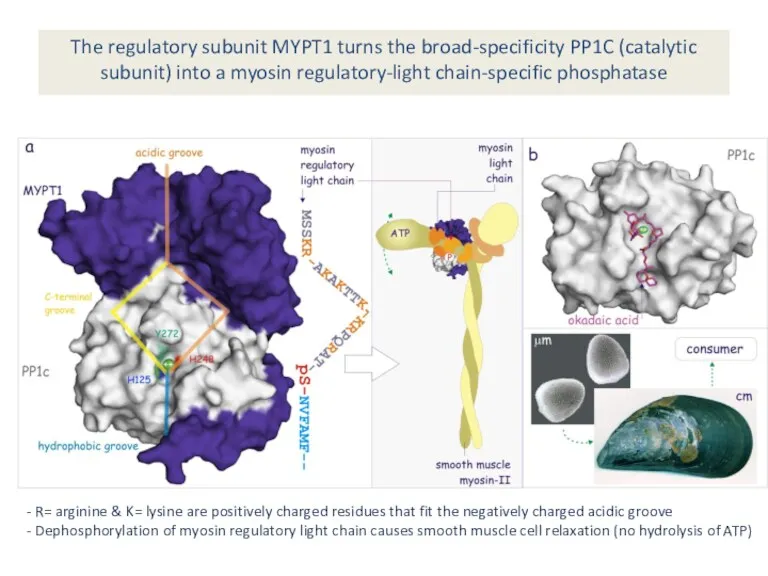
- 34. The regulatory subunit MYPT1 turns the broad-specificity PP1C (catalytic subunit) into a myosin regulatory-light chain-specific phosphatase
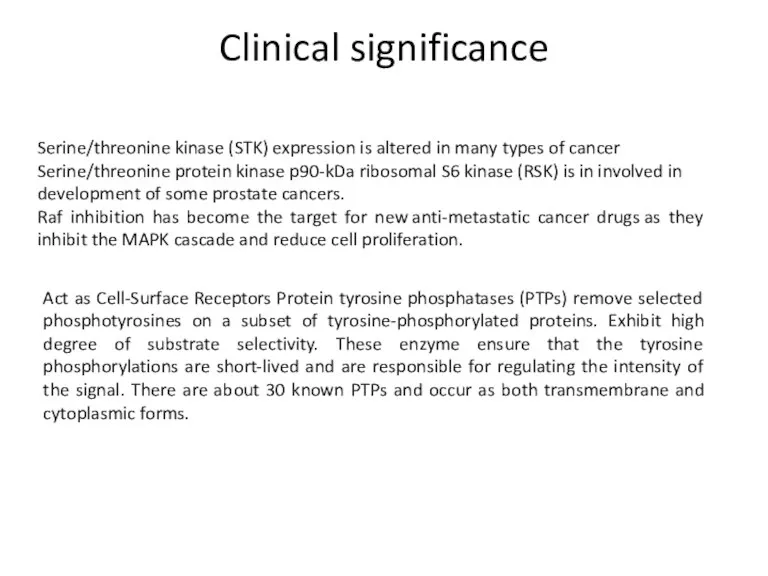
- 35. Clinical significance Serine/threonine kinase (STK) expression is altered in many types of cancer Serine/threonine protein kinase
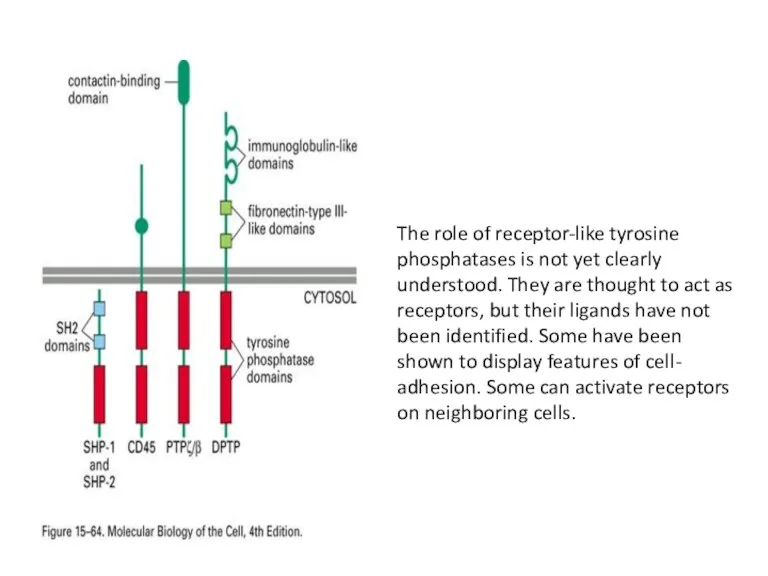
- 36. The role of receptor-like tyrosine phosphatases is not yet clearly understood. They are thought to act
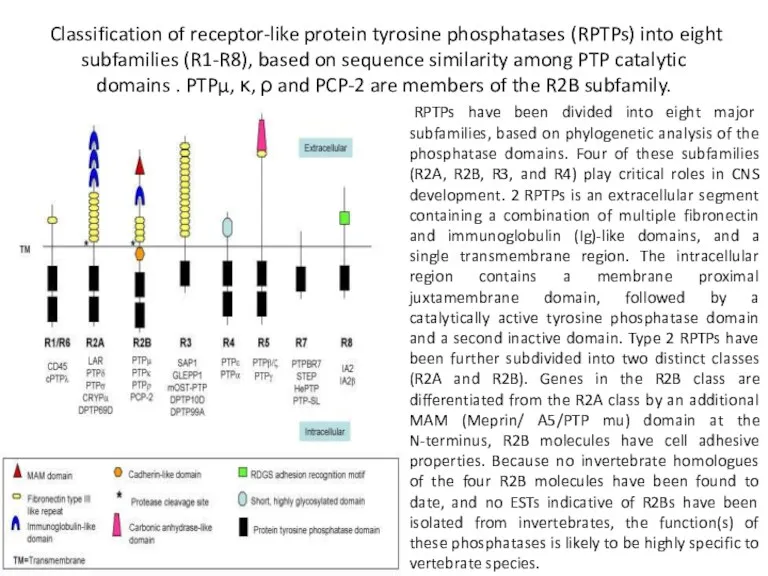
- 37. Classification of receptor-like protein tyrosine phosphatases (RPTPs) into eight subfamilies (R1-R8), based on sequence similarity among
- 38. Receptor guanylyl cyclases
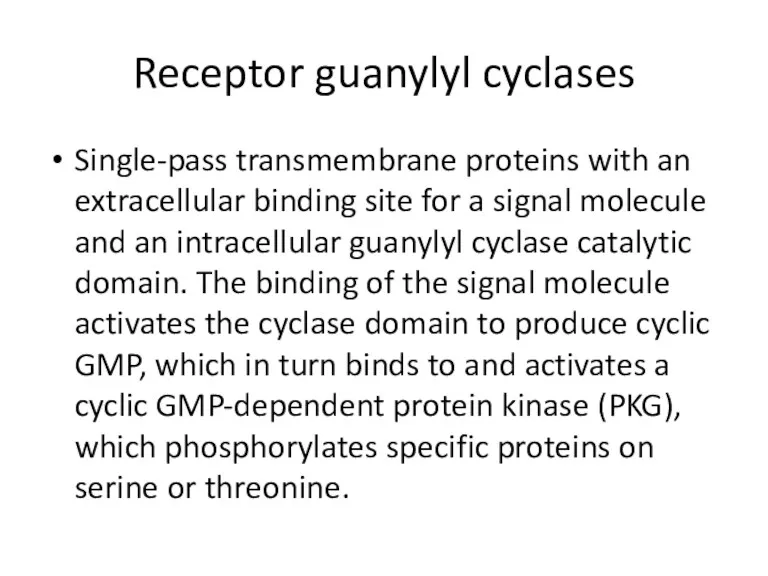
- 39. Receptor guanylyl cyclases Single-pass transmembrane proteins with an extracellular binding site for a signal molecule and
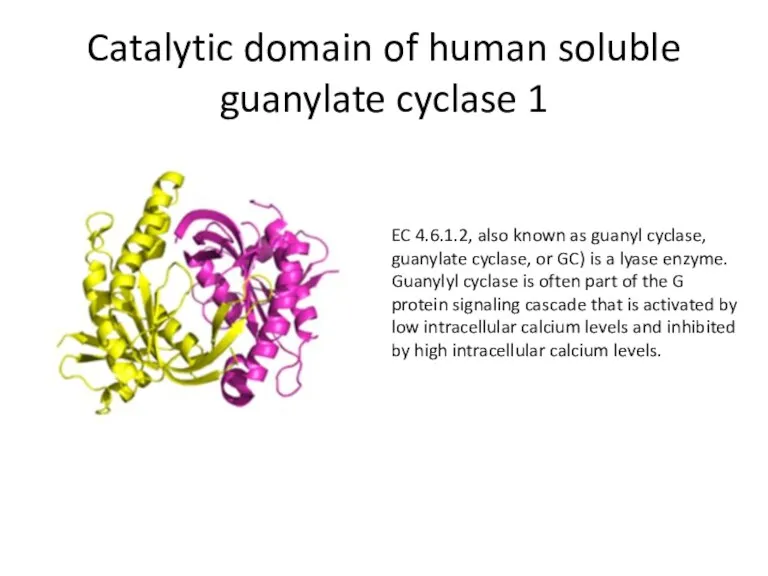
- 40. Catalytic domain of human soluble guanylate cyclase 1 EC 4.6.1.2, also known as guanyl cyclase, guanylate
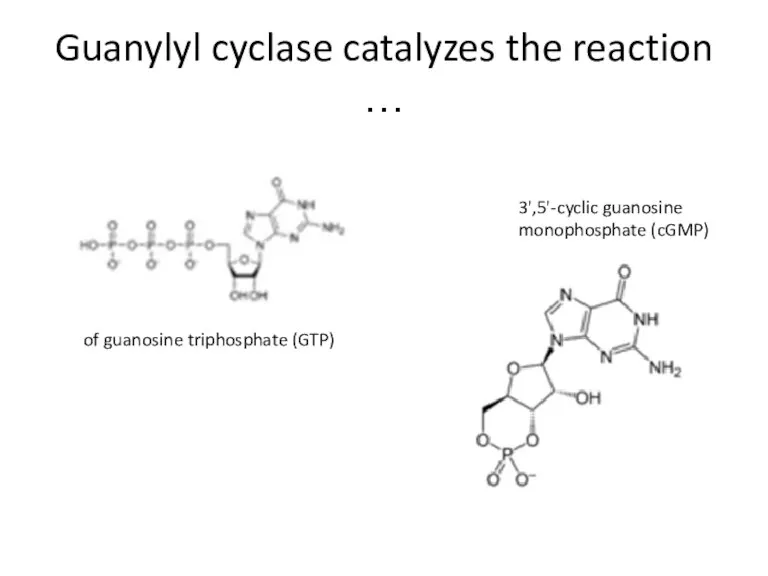
- 41. Guanylyl cyclase catalyzes the reaction … of guanosine triphosphate (GTP) 3',5'-cyclic guanosine monophosphate (cGMP)
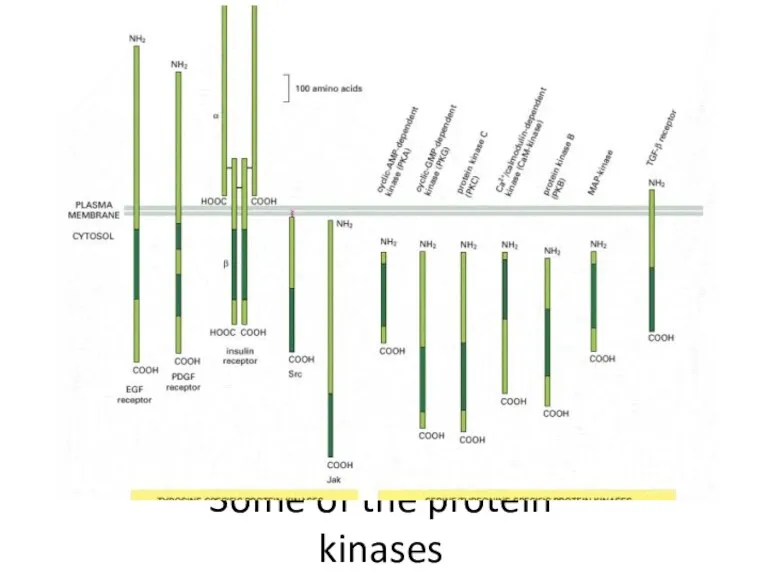
- 42. Some of the protein kinases
- 43. Histidine-kinase-associated receptors
- 44. Histidine-kinase-associated receptors Activate a “two-component” signaling pathway in which the kinase phosphorylates itself on histidine and
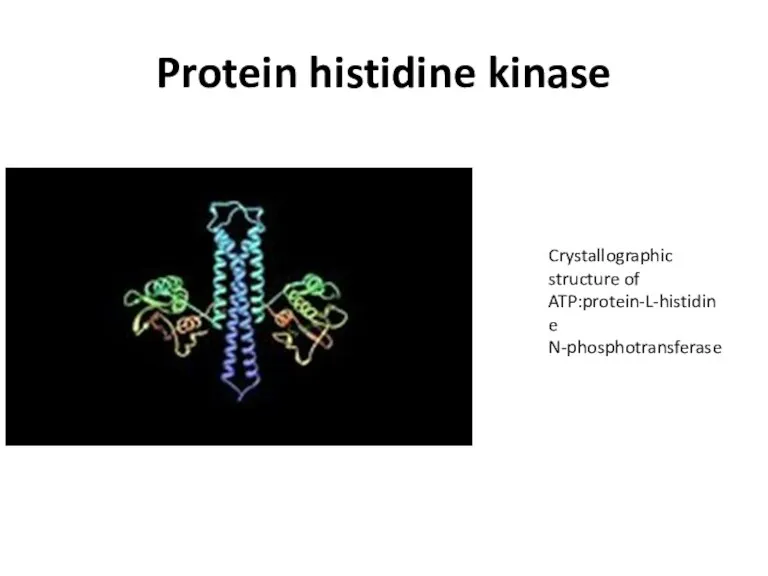
- 45. Protein histidine kinase Crystallographic structure of ATP:protein-L-histidine N-phosphotransferase
- 46. Multifunctional, typically transmembrane, proteins of the transferase class of enzymes that play a role insignal transduction
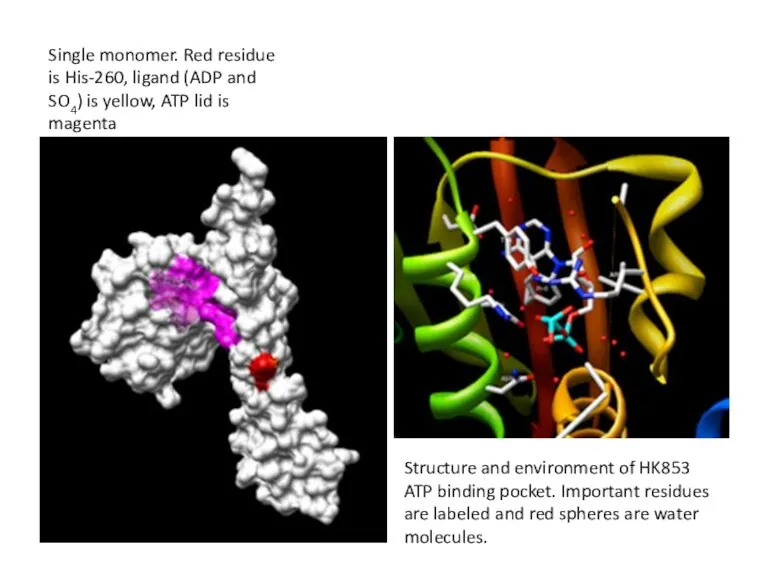
- 47. Single monomer. Red residue is His-260, ligand (ADP and SO4) is yellow, ATP lid is magenta
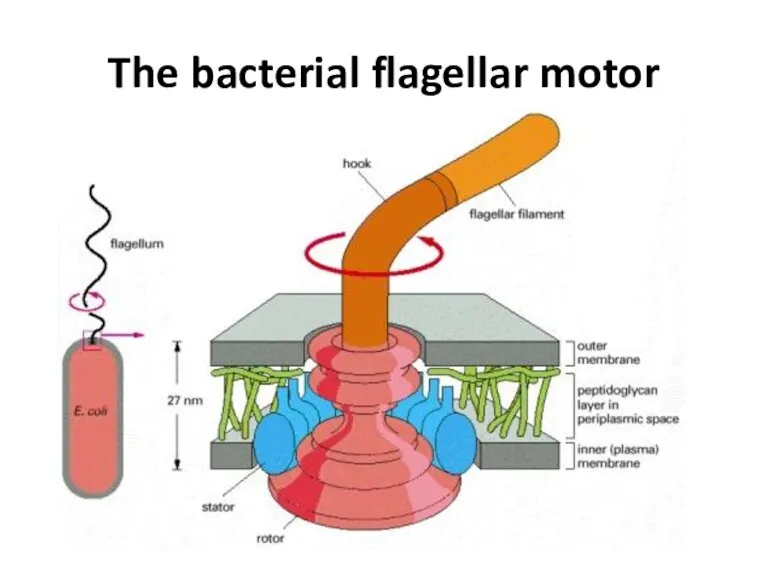
- 48. The bacterial flagellar motor
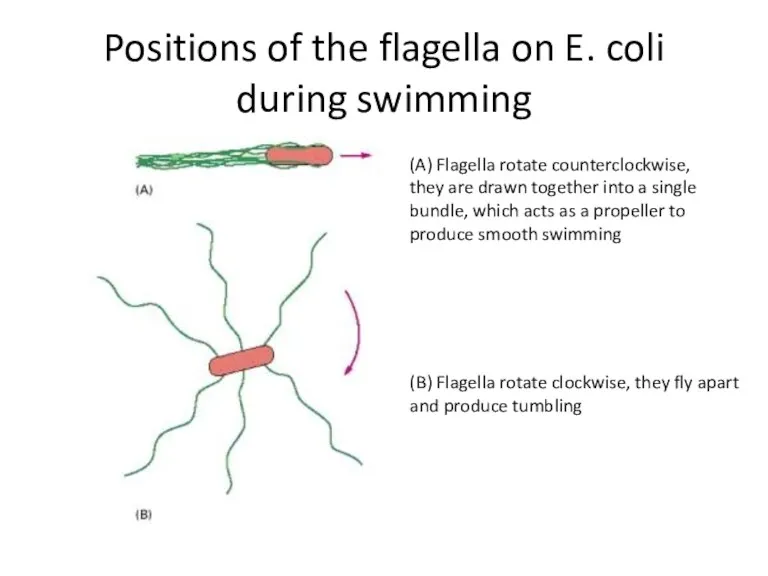
- 49. Positions of the flagella on E. coli during swimming (A) Flagella rotate counterclockwise, they are drawn
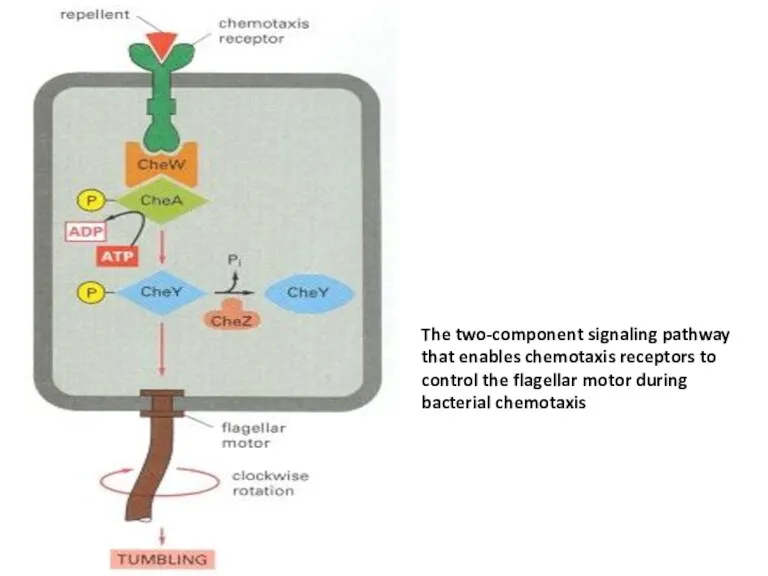
- 50. The two-component signaling pathway that enables chemotaxis receptors to control the flagellar motor during bacterial chemotaxis
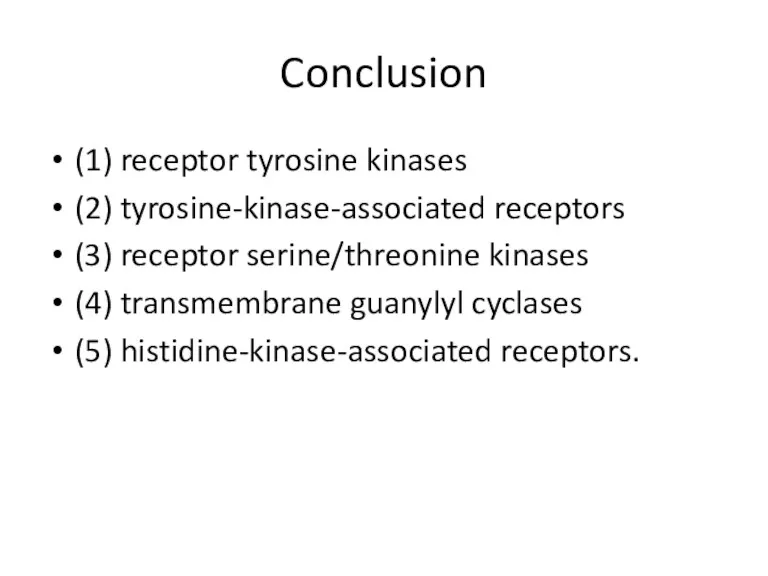
- 51. Conclusion (1) receptor tyrosine kinases (2) tyrosine-kinase-associated receptors (3) receptor serine/threonine kinases (4) transmembrane guanylyl cyclases
- 52. Some transmembrane tyrosine phosphatases, which remove phosphate from phosphotyrosine side chains of specific proteins, are thought
- 53. Tyrosine-kinase-associated receptors depend on various cytoplasmic tyrosine kinases for their action. These kinases include members of
- 54. Bacterial chemotaxis is mediated by histidine-kinase-associated chemotaxis receptors. When activated by the binding of a repellent,
- 56. Скачать презентацию






 Harry Potter (game)
Harry Potter (game) Monsters, inc
Monsters, inc [w ] White, Well, Winter, Welcome Well, welcome white winter!
[w ] White, Well, Winter, Welcome Well, welcome white winter! New Year
New Year My school
My school Методика обучения иностранным языкам
Методика обучения иностранным языкам Changes in the system of the english vocabulary
Changes in the system of the english vocabulary My ideal school
My ideal school My future profession is advertising specialist
My future profession is advertising specialist Parts of speech
Parts of speech Bingo game numbers
Bingo game numbers The family centre of Kazan
The family centre of Kazan Conflicts
Conflicts Telling the time. 6 english+lego. Magic teaching
Telling the time. 6 english+lego. Magic teaching Animals. Down on the farm
Animals. Down on the farm Canada. Geography
Canada. Geography Употребление количественных местоимений. Методическая разработка
Употребление количественных местоимений. Методическая разработка London City
London City Conditional clauses. Type 2. Game
Conditional clauses. Type 2. Game Today is the … of ……
Today is the … of …… My ideal country
My ideal country Present Simple
Present Simple London tour
London tour Говорение. Стратегия выполнения, полный разбор задания, схема ответа
Говорение. Стратегия выполнения, полный разбор задания, схема ответа Money and banking
Money and banking Типичные ошибки русскоговорящих в английском
Типичные ошибки русскоговорящих в английском Adjectives Opposites
Adjectives Opposites Ireland - Dublin
Ireland - Dublin