Содержание
- 2. I) Molecular Bonding B) Atom's Electronic Structure – Atomic Orbitals C) Valence Bond Theory: localized electrons
- 3. PREVIOUSLY ON From Elements to Molecules
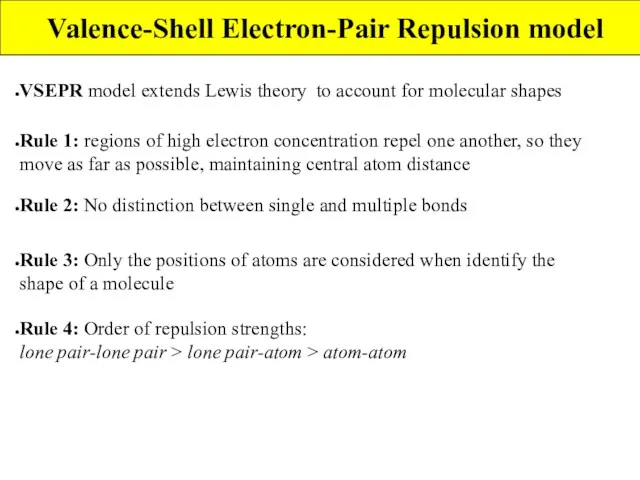
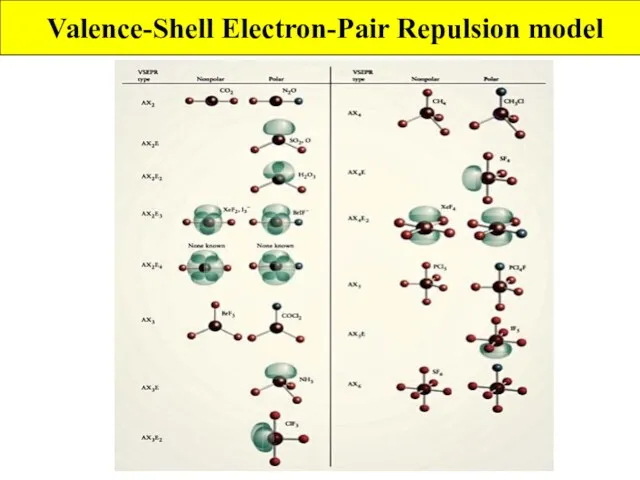
- 4. Valence-Shell Electron-Pair Repulsion model VSEPR model extends Lewis theory to account for molecular shapes Rule 1:
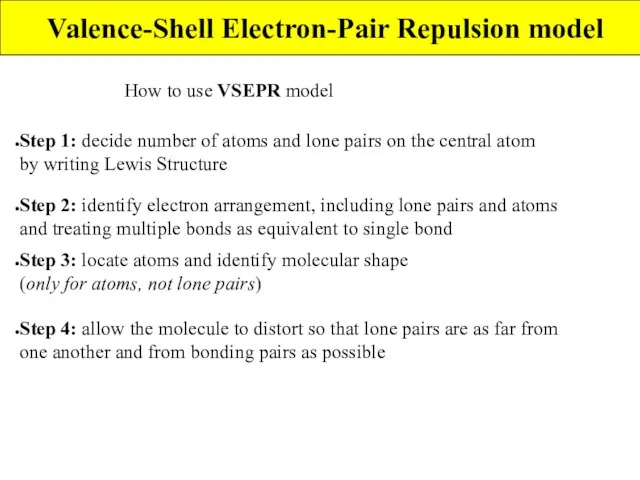
- 5. Valence-Shell Electron-Pair Repulsion model How to use VSEPR model Step 1: decide number of atoms and
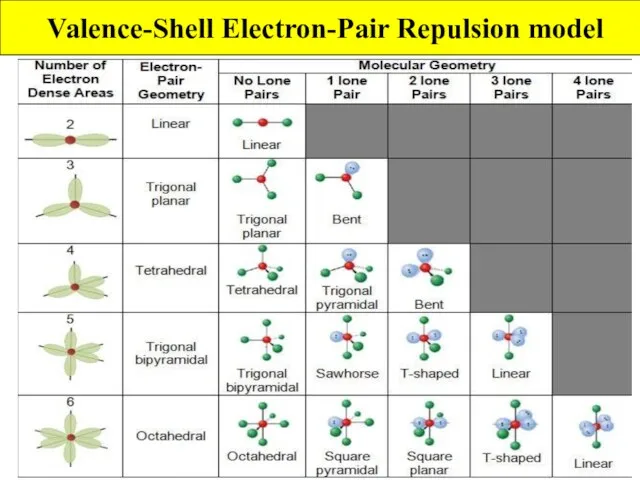
- 6. Valence-Shell Electron-Pair Repulsion model
- 7. Valence-Shell Electron-Pair Repulsion model VSEPR and Polar Molecules What is a polar molecule? non zero dipole
- 8. Valence-Shell Electron-Pair Repulsion model
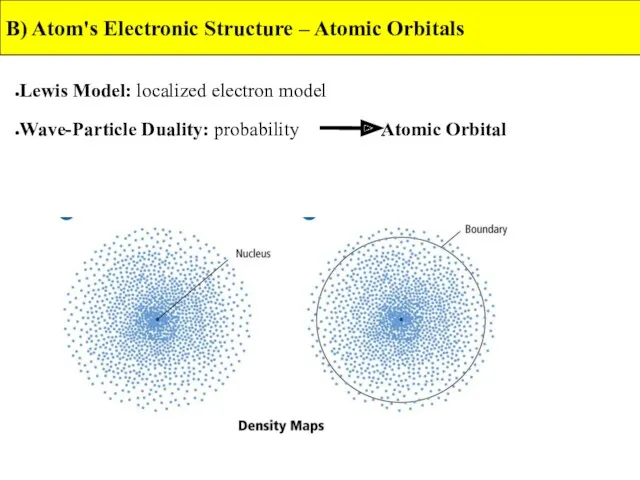
- 9. B) Atom's Electronic Structure – Atomic Orbitals Lewis Model: localized electron model Wave-Particle Duality: probability Atomic
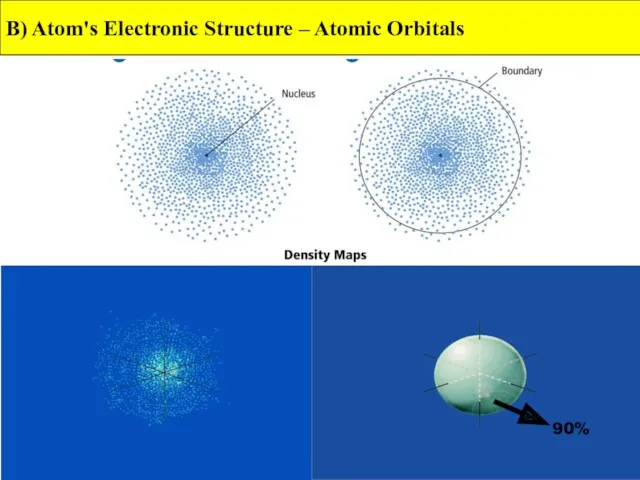
- 10. B) Atom's Electronic Structure – Atomic Orbitals 90%
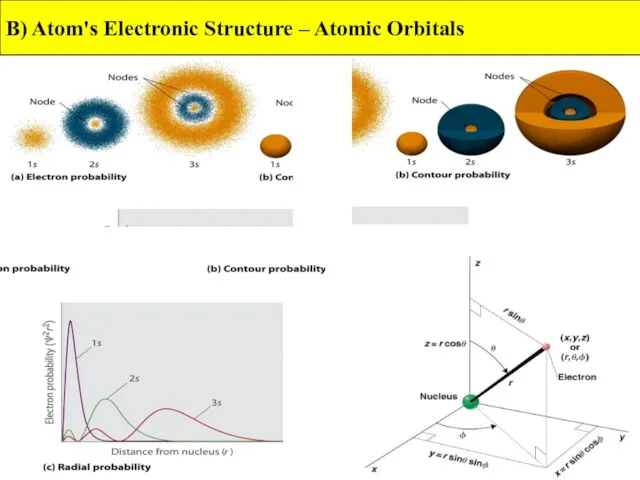
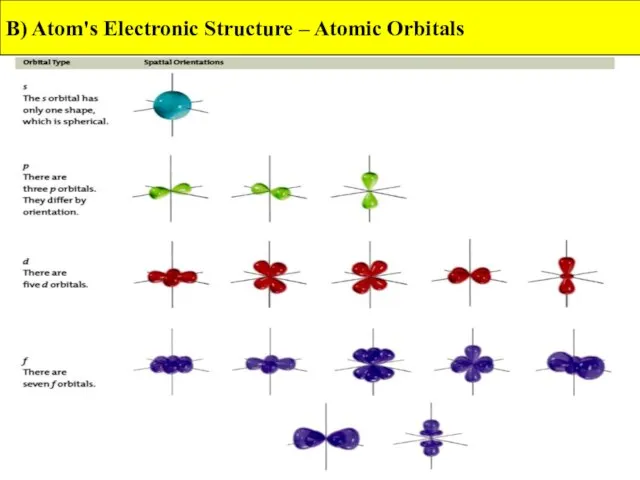
- 11. B) Atom's Electronic Structure – Atomic Orbitals
- 12. B) Atom's Electronic Structure – Atomic Orbitals
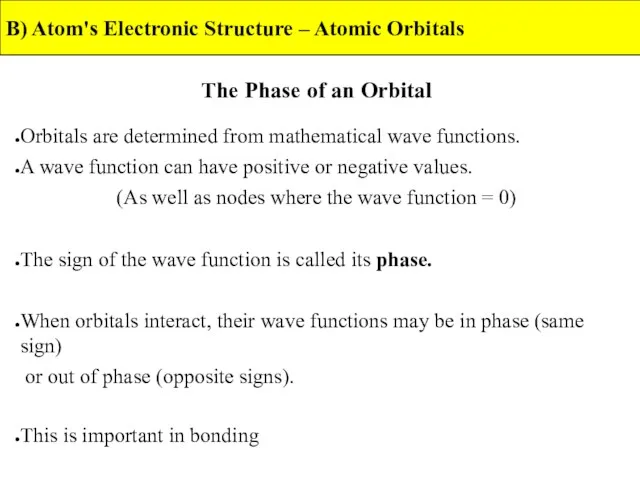
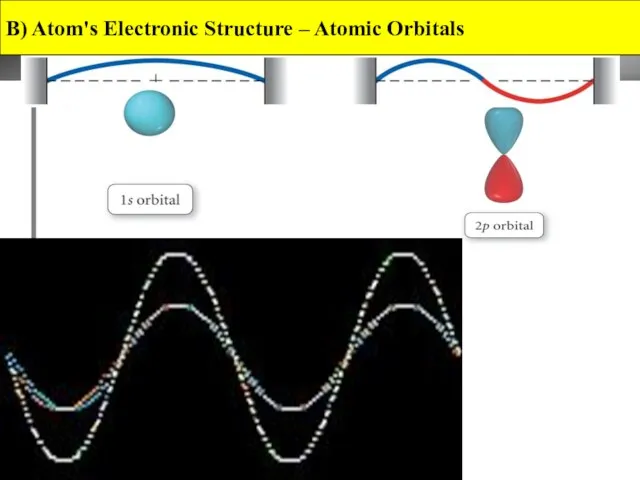
- 13. The Phase of an Orbital Orbitals are determined from mathematical wave functions. A wave function can
- 14. B) Atom's Electronic Structure – Atomic Orbitals
- 15. The basic principle of VB theory The space formed by the overlapping orbitals can accommodate a
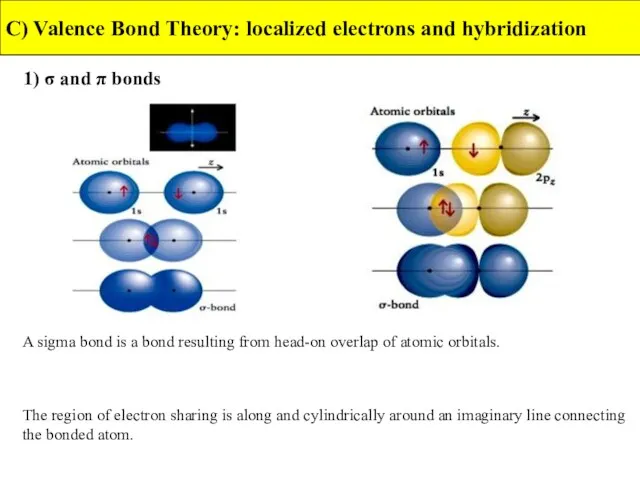
- 16. 1) σ- and π- bonds C) Valence Bond Theory: localized electrons and hybridization In valence-bond theory,
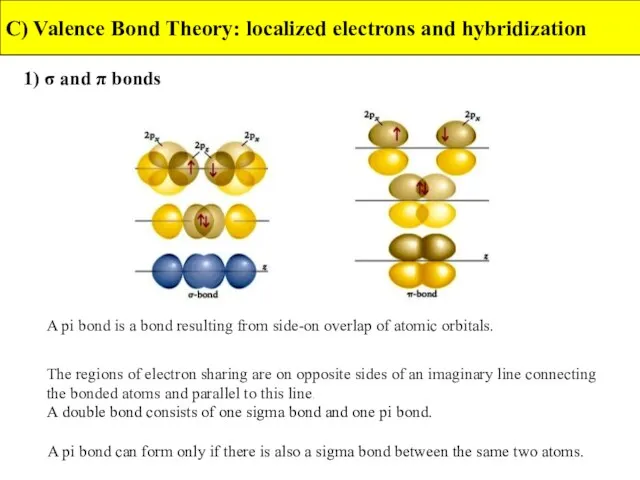
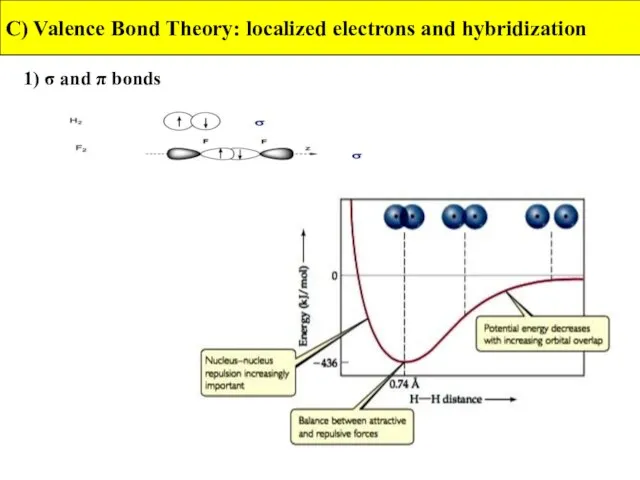
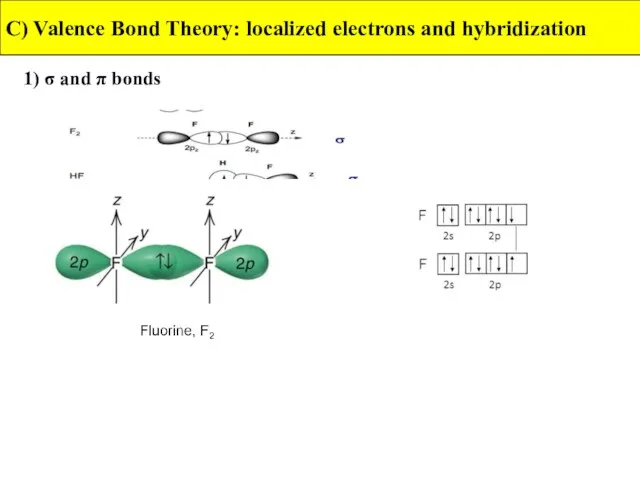
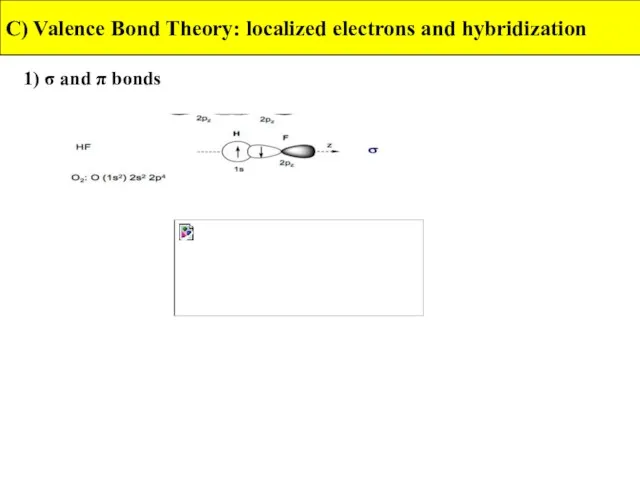
- 17. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization A sigma bond
- 18. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization A pi bond
- 19. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization
- 20. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization
- 21. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization
- 22. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization
- 23. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization Sample Problem Use
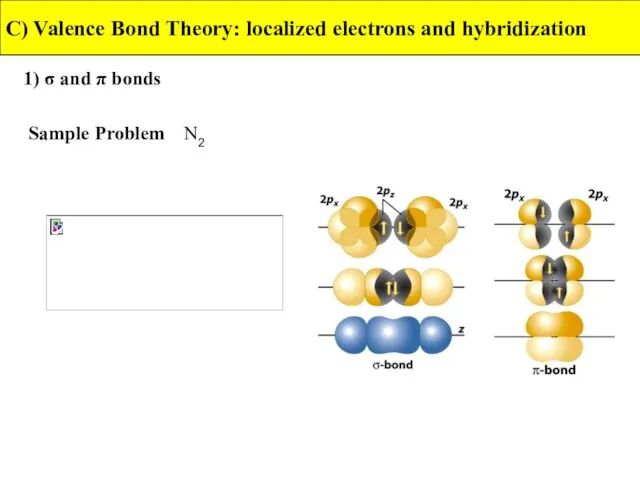
- 24. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization Sample Problem N2
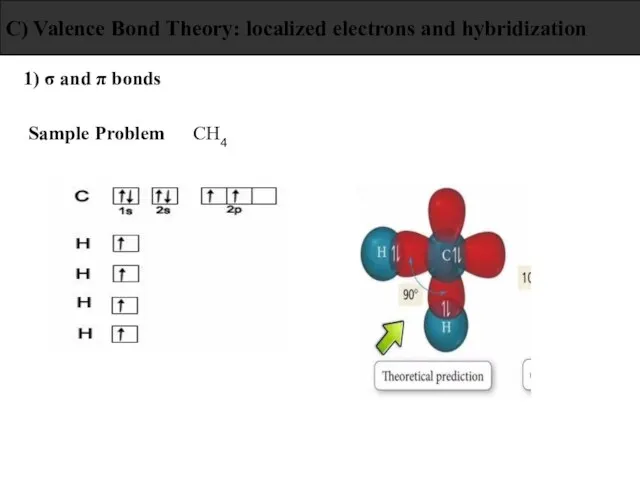
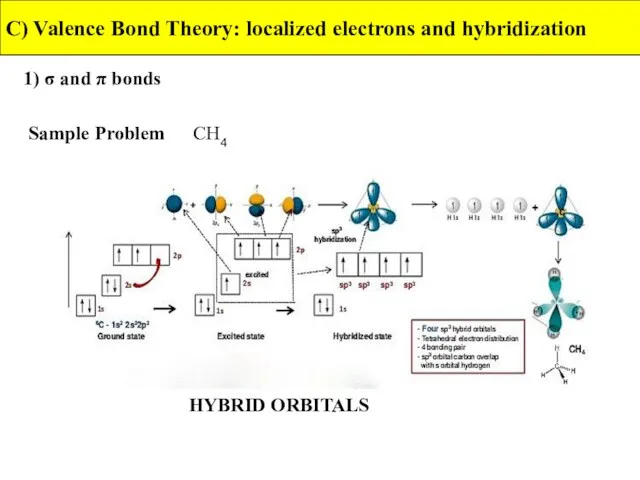
- 25. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization Sample Problem CH4
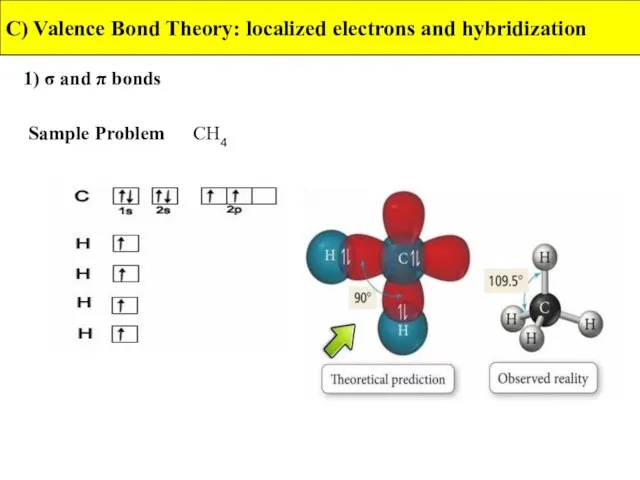
- 26. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization Sample Problem CH4
- 27. 1) σ and π bonds C) Valence Bond Theory: localized electrons and hybridization Sample Problem CH4
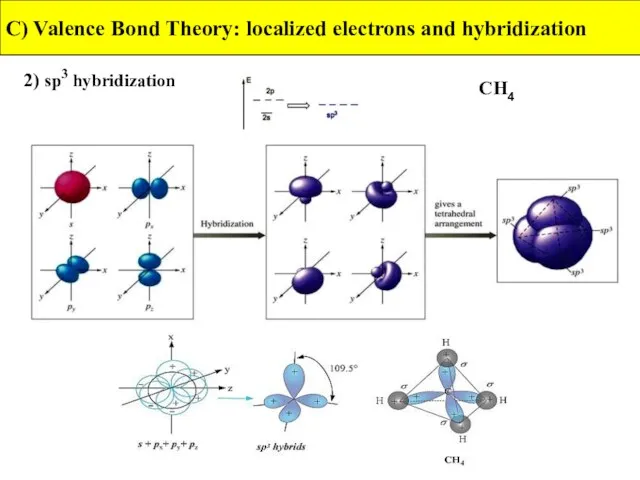
- 28. 2) sp3 hybridization C) Valence Bond Theory: localized electrons and hybridization CH4
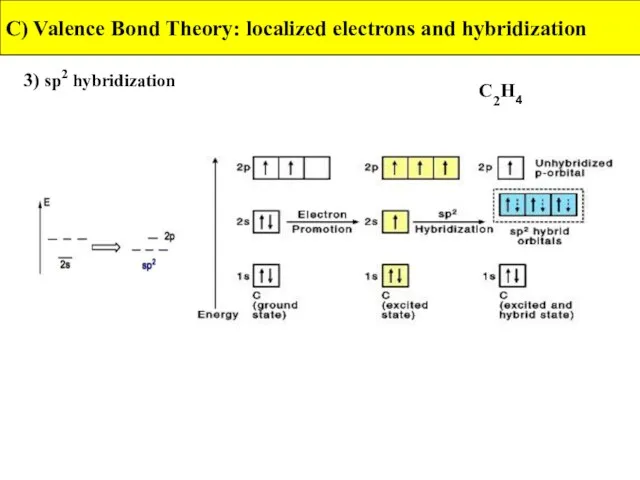
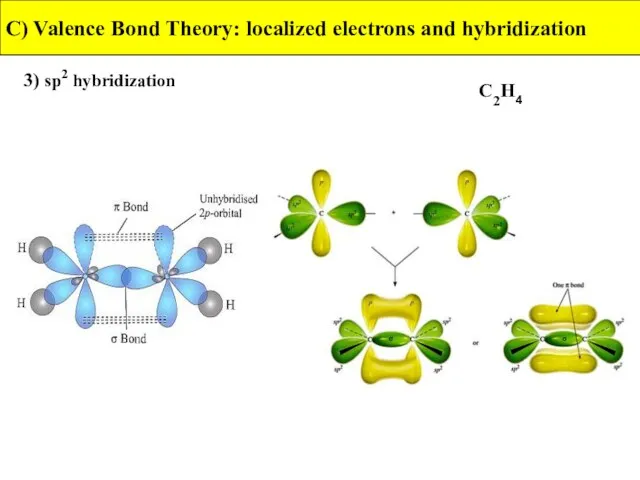
- 29. 3) sp2 hybridization C) Valence Bond Theory: localized electrons and hybridization C2H4
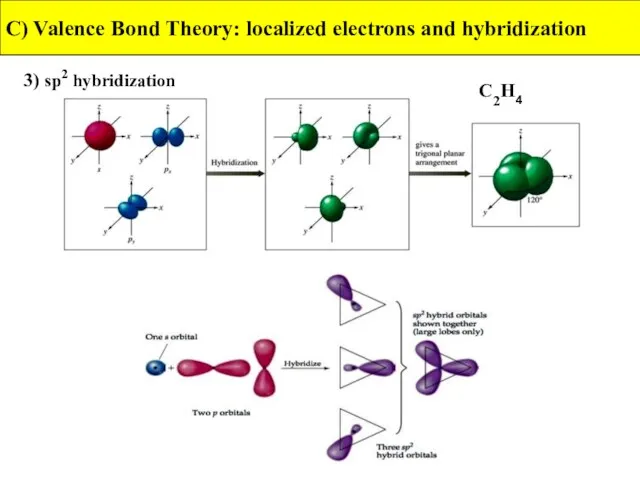
- 30. 3) sp2 hybridization C) Valence Bond Theory: localized electrons and hybridization C2H4
- 31. 3) sp2 hybridization C) Valence Bond Theory: localized electrons and hybridization C2H4
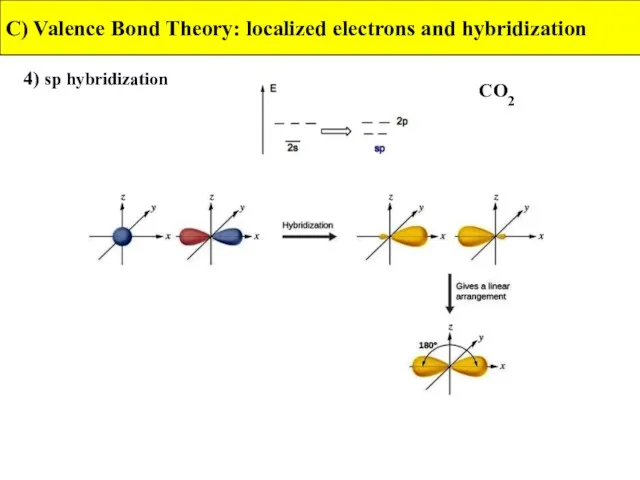
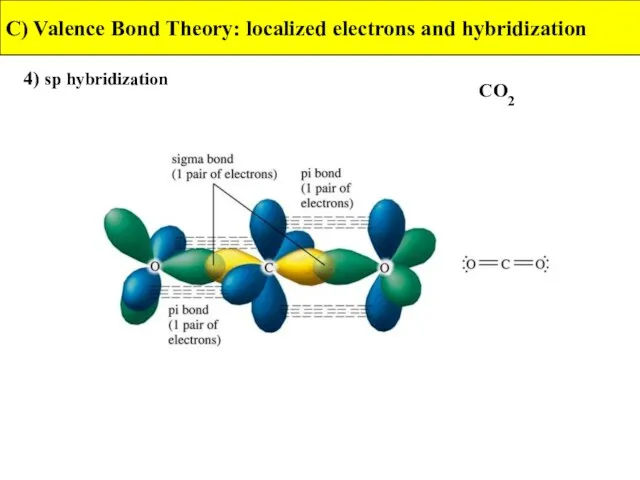
- 32. 4) sp hybridization C) Valence Bond Theory: localized electrons and hybridization CO2
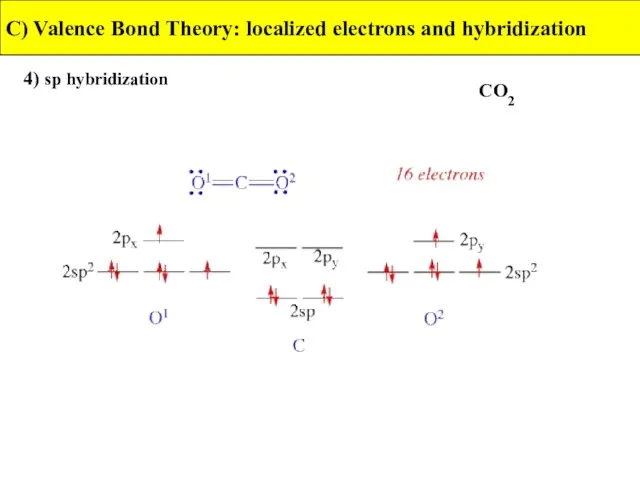
- 33. 4) sp hybridization C) Valence Bond Theory: localized electrons and hybridization CO2
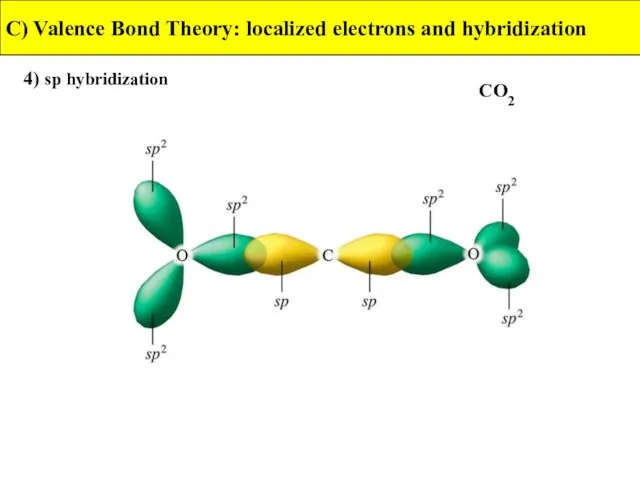
- 34. 4) sp hybridization C) Valence Bond Theory: localized electrons and hybridization CO2
- 35. 4) sp hybridization C) Valence Bond Theory: localized electrons and hybridization CO2
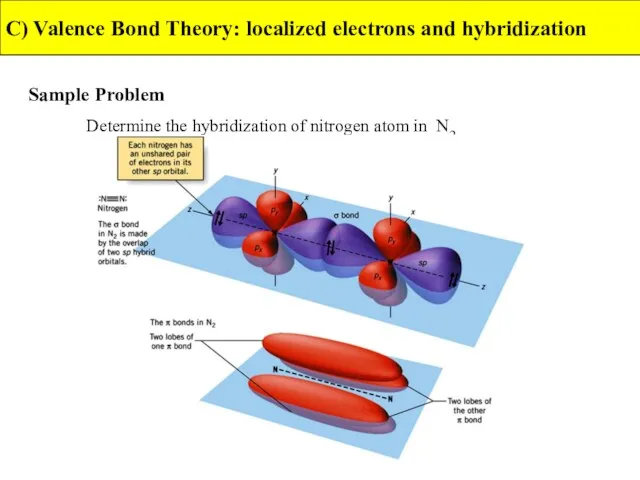
- 36. C) Valence Bond Theory: localized electrons and hybridization Sample Problem Determine the hybridization of nitrogen atom
- 37. C) Valence Bond Theory: localized electrons and hybridization Sample Problem Determine the hybridization of nitrogen atom
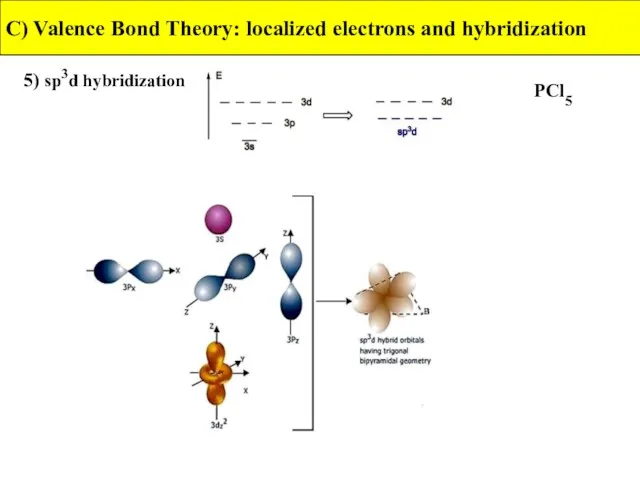
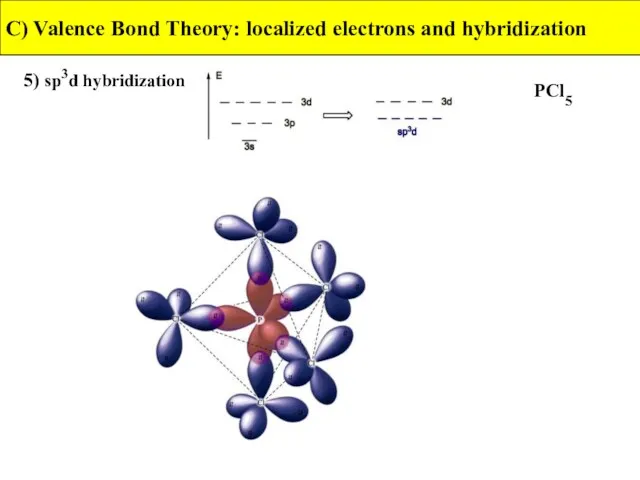
- 38. 5) sp3d hybridization C) Valence Bond Theory: localized electrons and hybridization PCl5
- 39. 5) sp3d hybridization C) Valence Bond Theory: localized electrons and hybridization PCl5
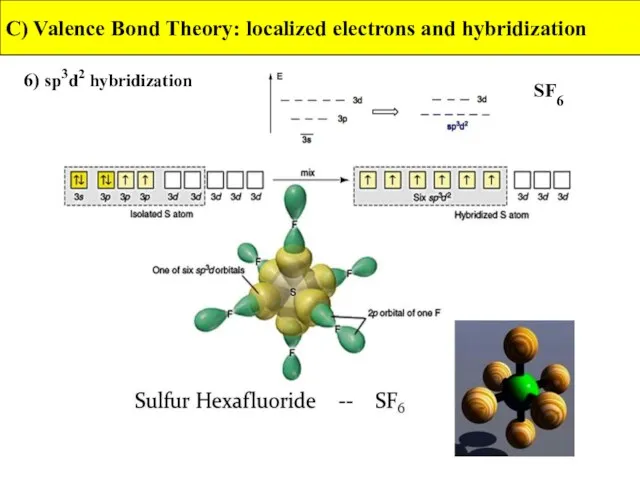
- 40. 6) sp3d2 hybridization C) Valence Bond Theory: localized electrons and hybridization SF6
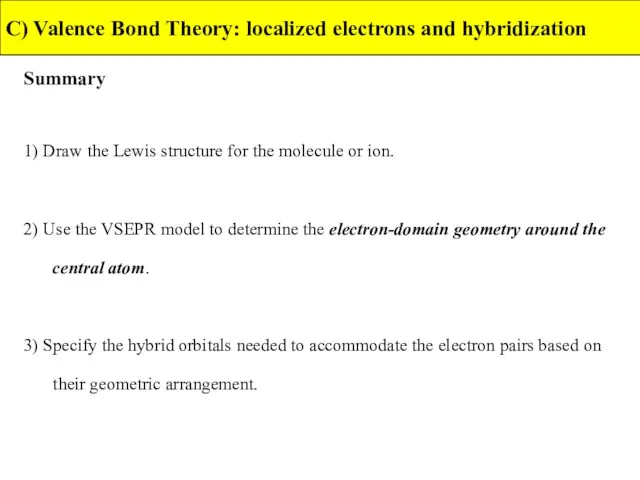
- 41. Summary C) Valence Bond Theory: localized electrons and hybridization 1) Draw the Lewis structure for the
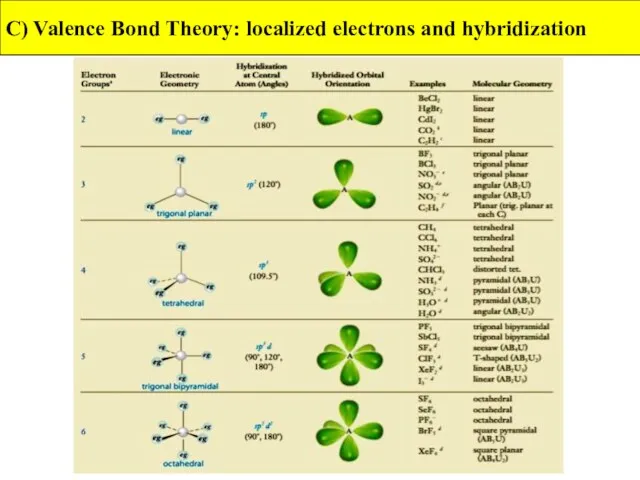
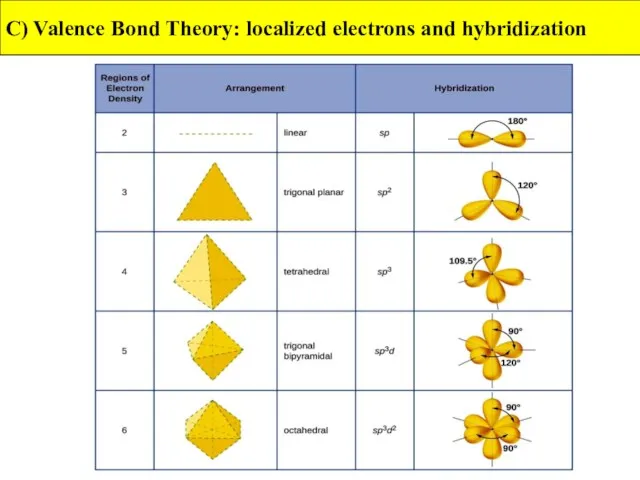
- 42. C) Valence Bond Theory: localized electrons and hybridization
- 43. C) Valence Bond Theory: localized electrons and hybridization
- 44. C) Valence Bond Theory: localized electrons and hybridization Weakeness Bonding Energies Localized electrons: mesomery not explained
- 46. Скачать презентацию






 Кинематика
Кинематика Закономерности механического движения
Закономерности механического движения Электрический ток в полупроводниках
Электрический ток в полупроводниках Атмосферное давление
Атмосферное давление Применение фотоэффекта
Применение фотоэффекта Рульове управління та гальмівна система БТР-80
Рульове управління та гальмівна система БТР-80 Резание металла слесарной ножовкой
Резание металла слесарной ножовкой Изобретение радио
Изобретение радио Закон Ома. Сопротивление. Что такое электрический ток?
Закон Ома. Сопротивление. Что такое электрический ток?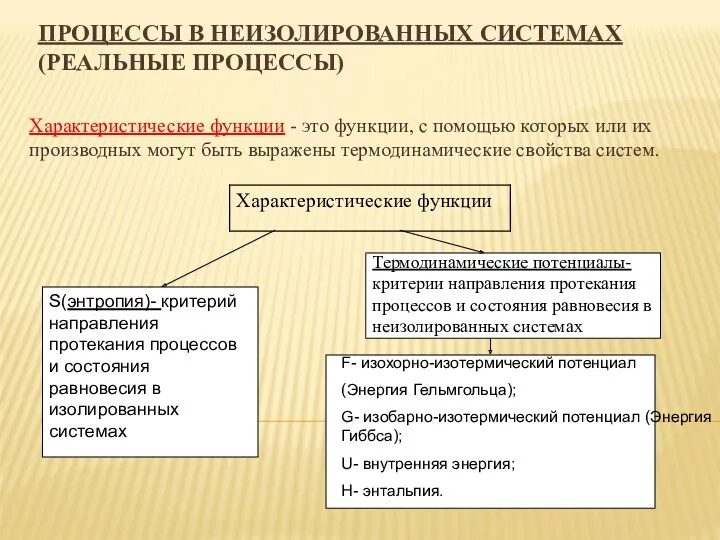 Процессы в неизолированных системах
Процессы в неизолированных системах Этапы развития ускорителей. Методы ускорения. Магнитная система ускорителей. Источники частиц. Синхротронное излучение
Этапы развития ускорителей. Методы ускорения. Магнитная система ускорителей. Источники частиц. Синхротронное излучение Текущий ремонт разъединителя РНДЗ-1-35/1000
Текущий ремонт разъединителя РНДЗ-1-35/1000 Лампочка. Принцип работы лампы накаливания
Лампочка. Принцип работы лампы накаливания Контрольно-измерительные приборы
Контрольно-измерительные приборы Влияние легирования на растворимость
Влияние легирования на растворимость Основы триботехники. Лекция 1
Основы триботехники. Лекция 1 Радіоактивний розпад елементів
Радіоактивний розпад елементів Восстановление корпусных деталей
Восстановление корпусных деталей Уравнение состояния идеального газа
Уравнение состояния идеального газа презентация по теме Кипение
презентация по теме Кипение Рентген сәулесімен ауруды анықтау
Рентген сәулесімен ауруды анықтау Электромагнитные волны и их свойства. Шкала электромагнитных волн
Электромагнитные волны и их свойства. Шкала электромагнитных волн Квазиравновесный конденсат поляритонов в GaAs микрорезонаторах в магнитном поле
Квазиравновесный конденсат поляритонов в GaAs микрорезонаторах в магнитном поле Загадки по физики
Загадки по физики Молекулярная физика
Молекулярная физика Контурные тепловые трубы в космической технике
Контурные тепловые трубы в космической технике Твердотельный лазер на основе кристалла Nd:YAG
Твердотельный лазер на основе кристалла Nd:YAG Актуальные вопросы подготовке к ЕГЭ по физике.
Актуальные вопросы подготовке к ЕГЭ по физике.