Содержание
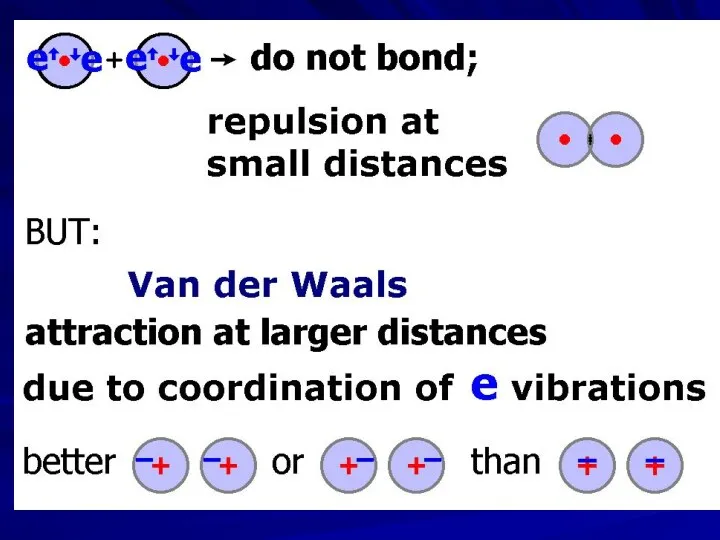
- 3. Johannes Diderik van der Waals (1837 – 1923) — Nobel Prize 1910 Fritz Wolfgang London (1900
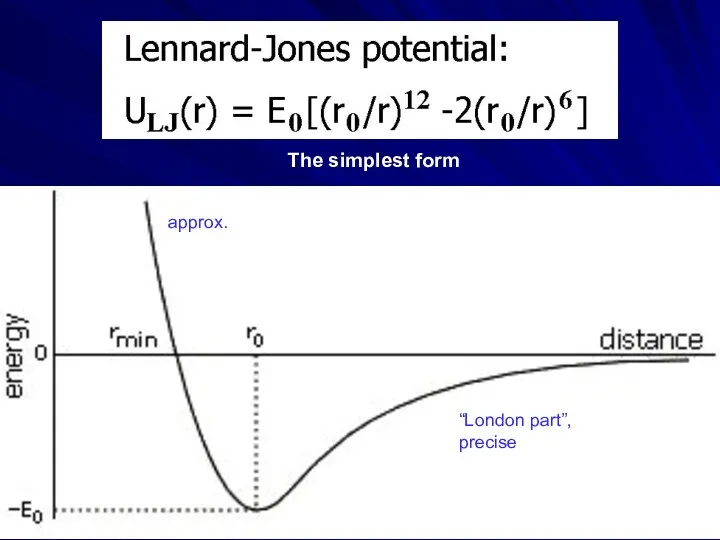
- 4. “London part”, precise approx. The simplest form
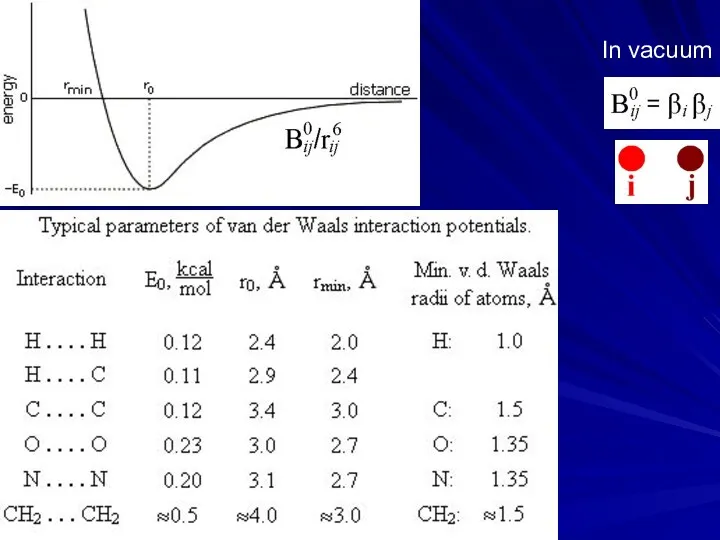
- 5. In vacuum
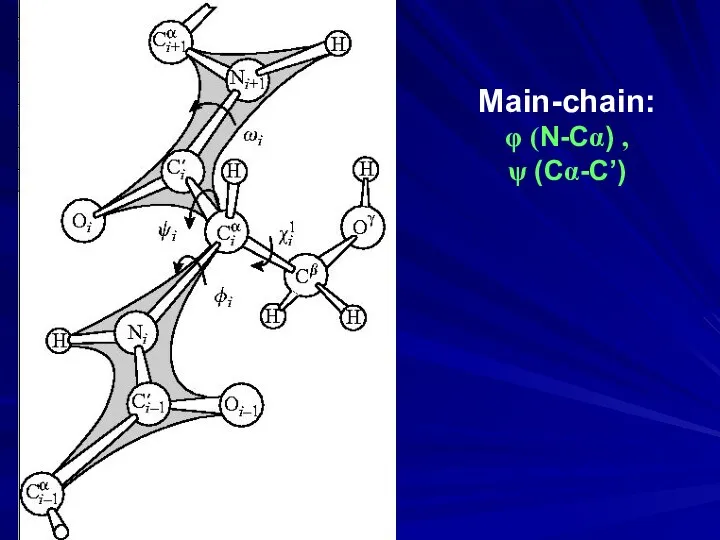
- 6. Main-chain: φ (N-Cα) , ψ (Cα-C’)
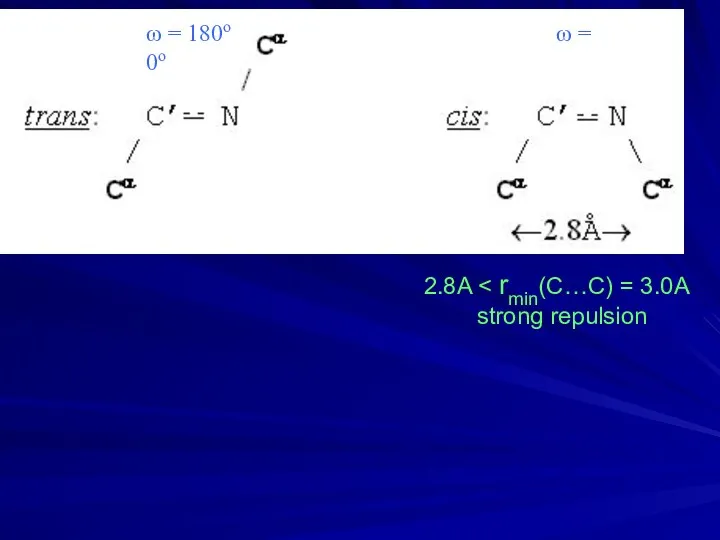
- 7. ω = 180ο ω = 0ο 2.8A strong repulsion
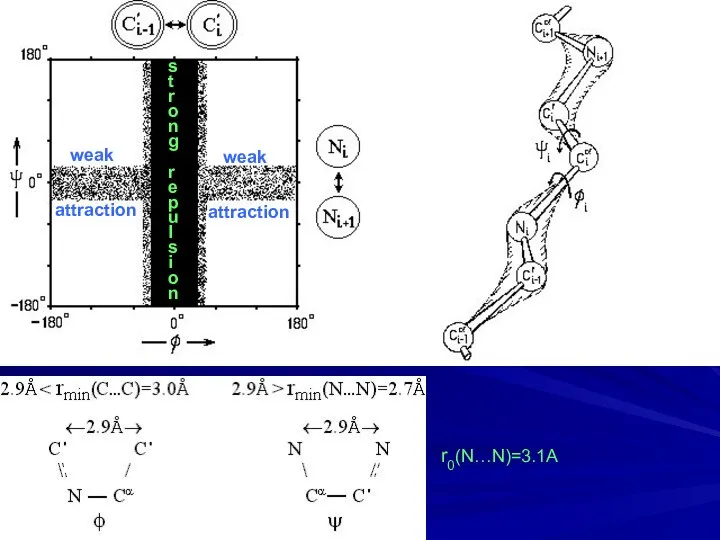
- 8. s t r o n g r e p u l s i o n weak
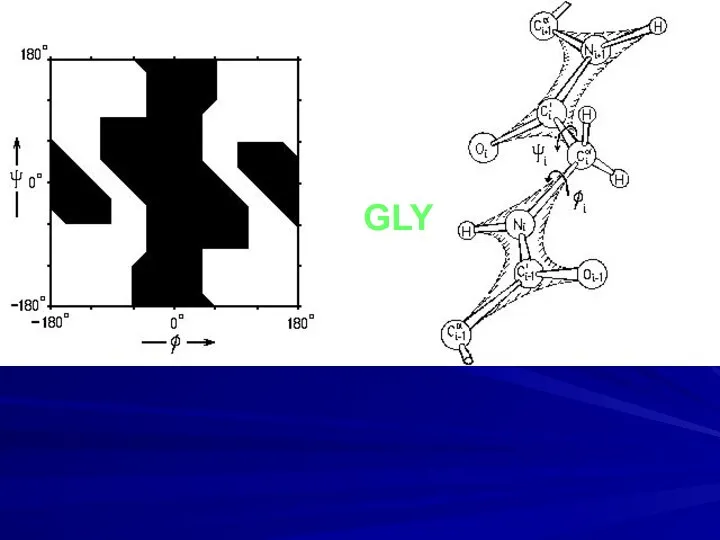
- 9. GLY
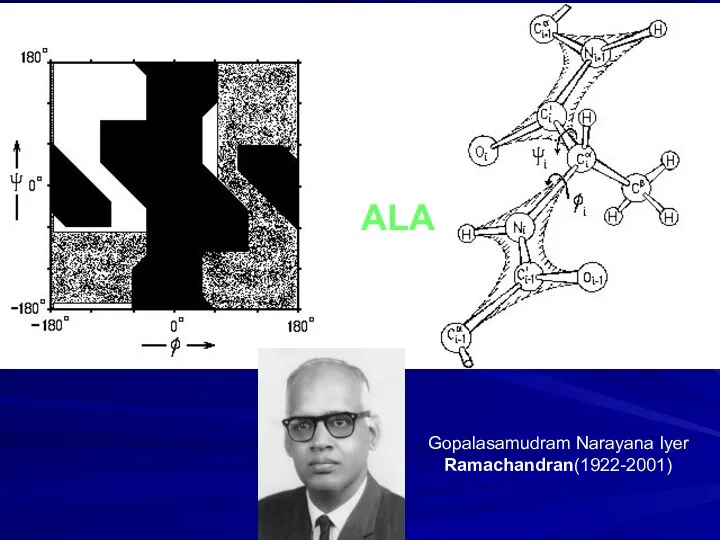
- 10. ALA Gopalasamudram Narayana Iyer Ramachandran(1922-2001)
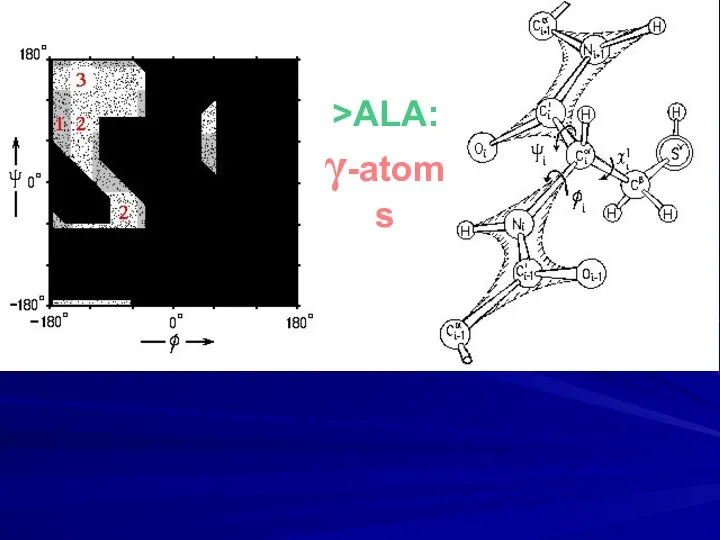
- 11. >ALA: γ-atoms
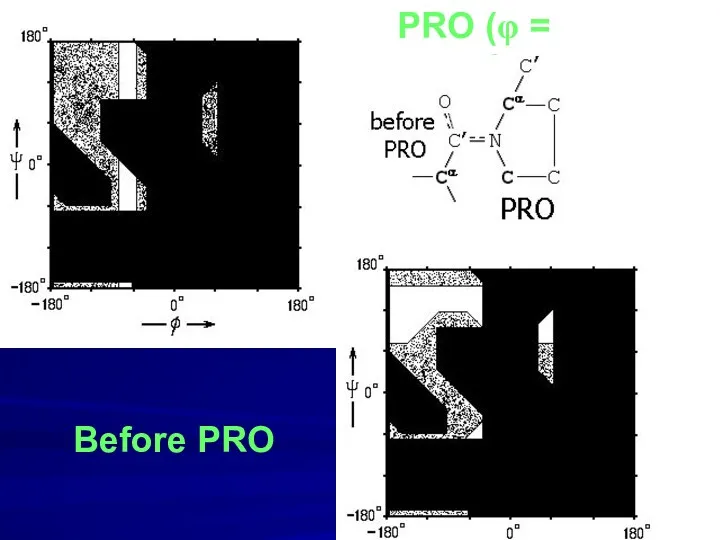
- 12. PRO (φ = -70o) Before PRO
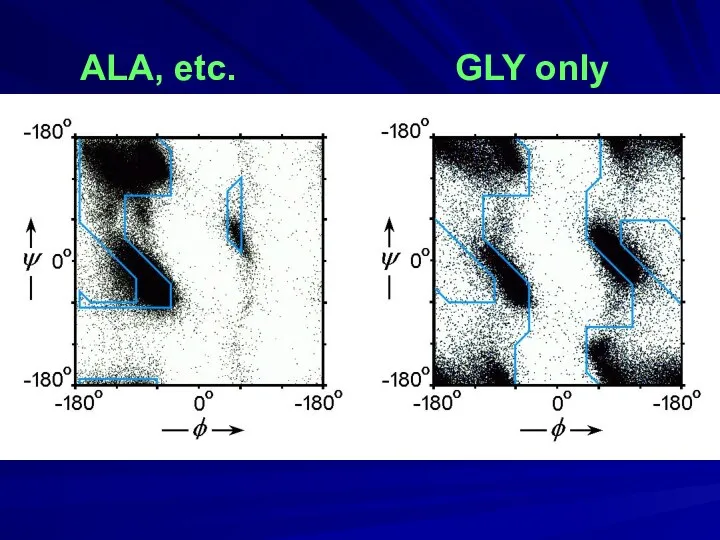
- 13. ALA, etc. GLY only
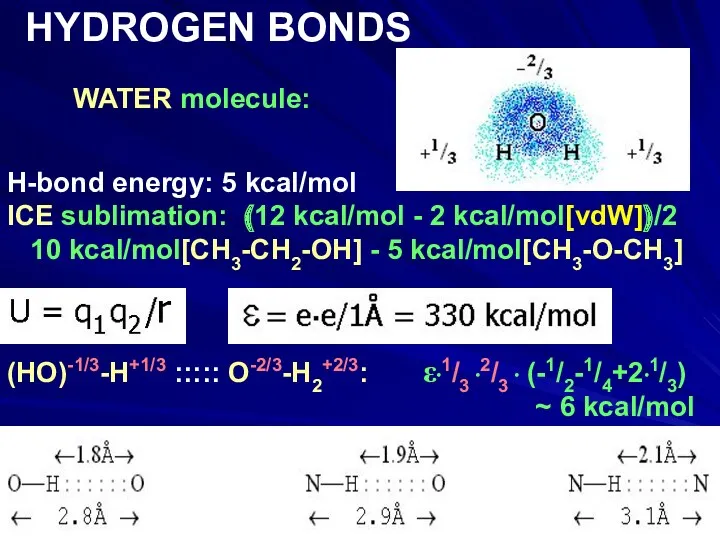
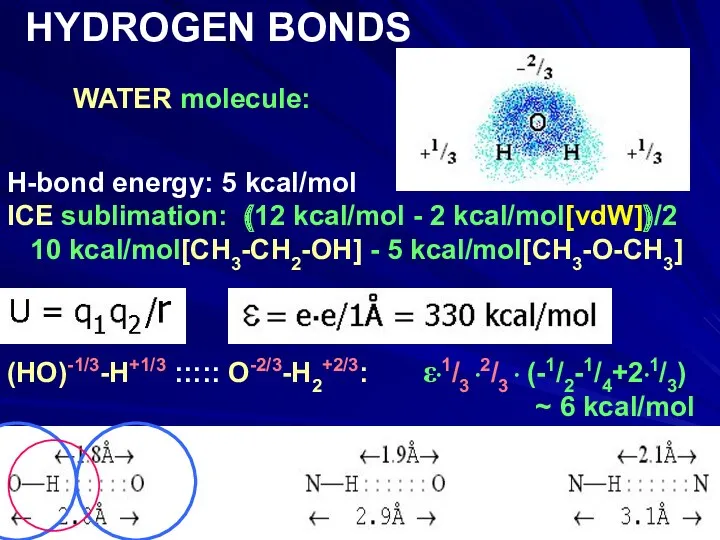
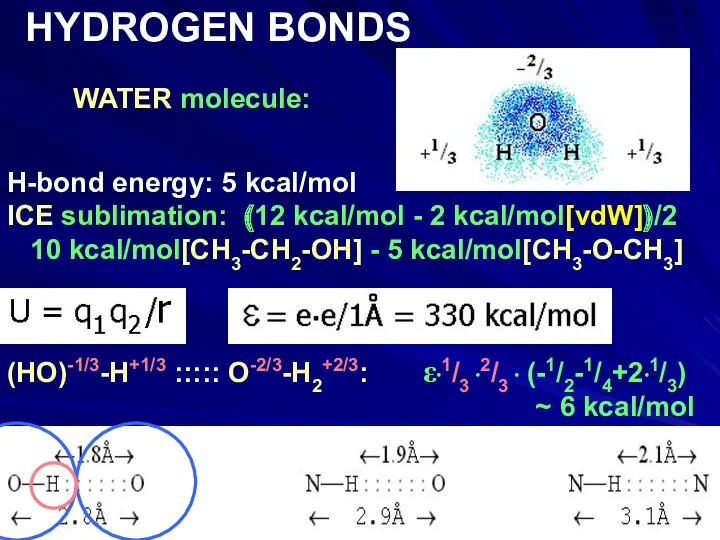
- 14. WATER molecule: HYDROGEN BONDS H-bond energy: 5 kcal/mol ICE sublimation: (12 kcal/mol - 2 kcal/mol[vdW])/2 10
- 15. WATER molecule: HYDROGEN BONDS H-bond energy: 5 kcal/mol ICE sublimation: (12 kcal/mol - 2 kcal/mol[vdW])/2 10
- 16. WATER molecule: HYDROGEN BONDS H-bond energy: 5 kcal/mol ICE sublimation: (12 kcal/mol - 2 kcal/mol[vdW])/2 10
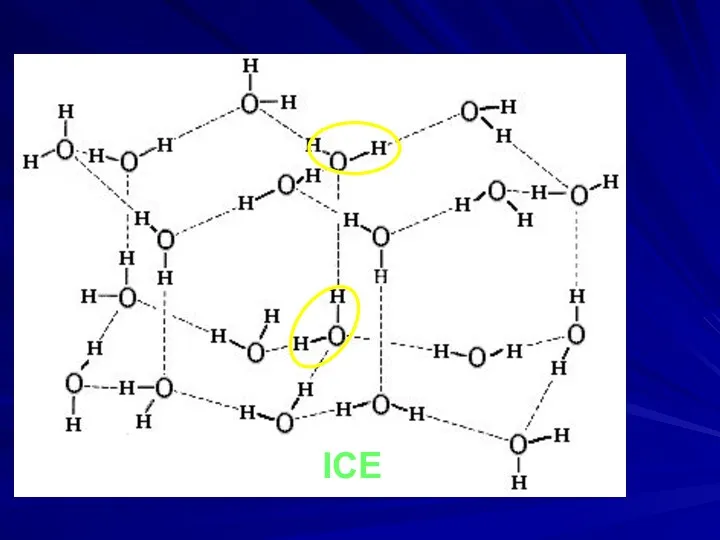
- 18. ICE
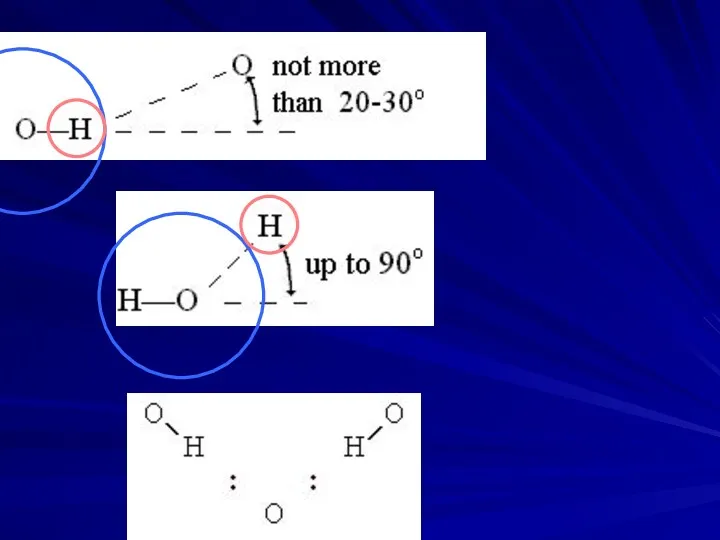
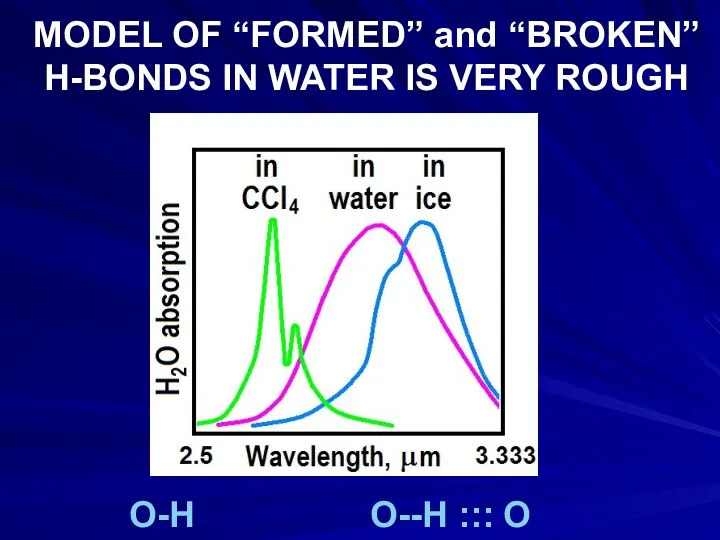
- 19. MODEL OF “FORMED” and “BROKEN” H-BONDS IN WATER IS VERY ROUGH O-H O--H ::: O
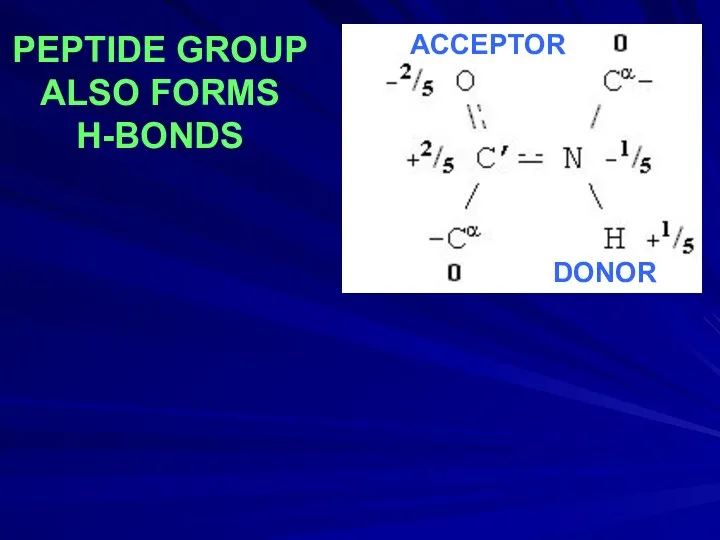
- 20. PEPTIDE GROUP ALSO FORMS H-BONDS DONOR ACCEPTOR
- 22. Скачать презентацию

 Гипотеза де Бройля. Комптоновская длина волны и длина волны де Бройля
Гипотеза де Бройля. Комптоновская длина волны и длина волны де Бройля Применение сообщающихся сосудов
Применение сообщающихся сосудов Исследовательская деятельность на уроках физики
Исследовательская деятельность на уроках физики Загальні відомості про насоси
Загальні відомості про насоси Субмикронная литография
Субмикронная литография Радиоактивность. Модели атомов. Опыт Резерфорда
Радиоактивность. Модели атомов. Опыт Резерфорда Адсорбция үдерісі
Адсорбция үдерісі Диагностирование системы смазки двигателя
Диагностирование системы смазки двигателя Линейные излучающие системы
Линейные излучающие системы Ядерная физика (Лекция 9)
Ядерная физика (Лекция 9) Графическое представление движения. Урок физики. 7 класс
Графическое представление движения. Урок физики. 7 класс Строение атома
Строение атома Цепные передачи
Цепные передачи Yüzey ve kompozi̇syon (9)
Yüzey ve kompozi̇syon (9) Lasers. Tutorial 2
Lasers. Tutorial 2 Презентация к уроку в 7 классе по теме Масса тела.Измерение массы тела с помощью весов
Презентация к уроку в 7 классе по теме Масса тела.Измерение массы тела с помощью весов Основные положения молекулярно-кинетической теории. Размеры молекул
Основные положения молекулярно-кинетической теории. Размеры молекул Сценарий урока в 9 классе Величина, характеризующие колебательное движение
Сценарий урока в 9 классе Величина, характеризующие колебательное движение Взаимодействие тел. Масса тела.
Взаимодействие тел. Масса тела. Фотоосновы. Экспозиция
Фотоосновы. Экспозиция Сила упругости. Закон Гука. 7 класс
Сила упругости. Закон Гука. 7 класс Открытый урок в 7 кл на тему Плавание тел
Открытый урок в 7 кл на тему Плавание тел Измерение размеров малых тел. Лабораторная работа
Измерение размеров малых тел. Лабораторная работа Тліючий розряд
Тліючий розряд Технические измерения. Допуски и посадки гладких цилиндрических соединений деталей (гцс)
Технические измерения. Допуски и посадки гладких цилиндрических соединений деталей (гцс) Гидравлический привод сцепления автомобиля Камаз
Гидравлический привод сцепления автомобиля Камаз Высокоточные системы навигации. Лекция №1.3
Высокоточные системы навигации. Лекция №1.3 ЖРД. Устройство и принцип действия, внутрикамерные процессы. (Лекция 2)
ЖРД. Устройство и принцип действия, внутрикамерные процессы. (Лекция 2)