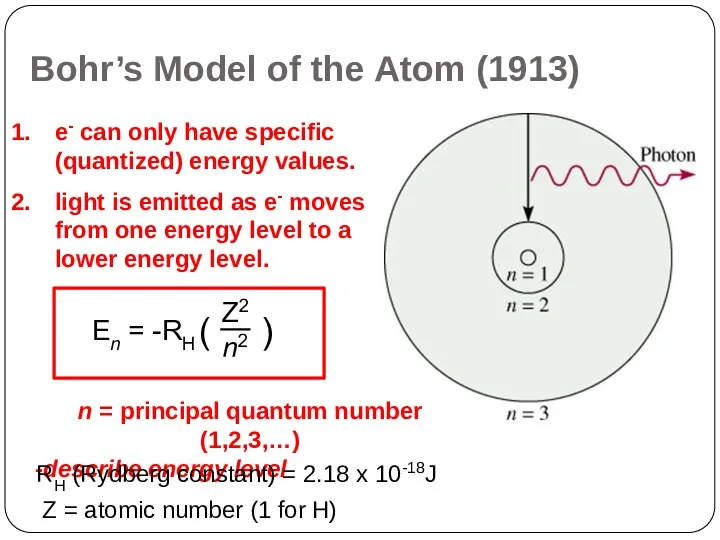
Bohr’s Model of the Atom (1913)
Electrons cannot have just any amount
of energy but can have only certain specified amount; i.e. the energy of an electron is quantized. The specified energy values for an atom are called its energy levels.
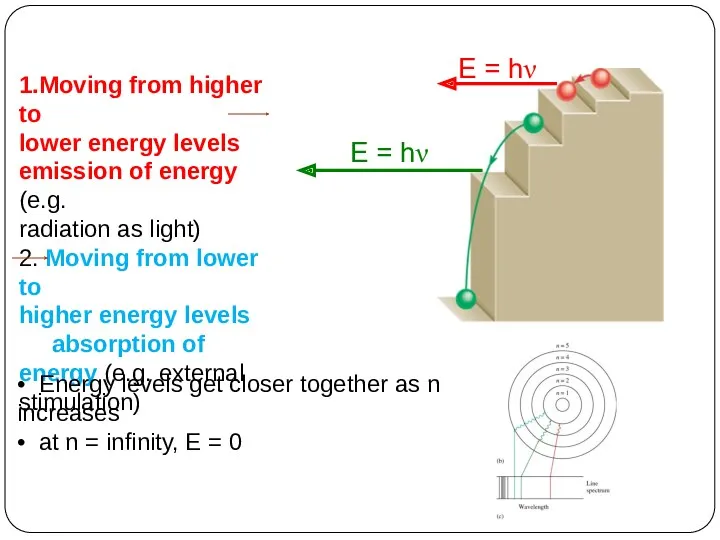
As an electron moves instantaneously from one energy level to another, there are no intermediate stages.
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922.
http://www.youtube.com/watch?v=Ic8OnvRonb0

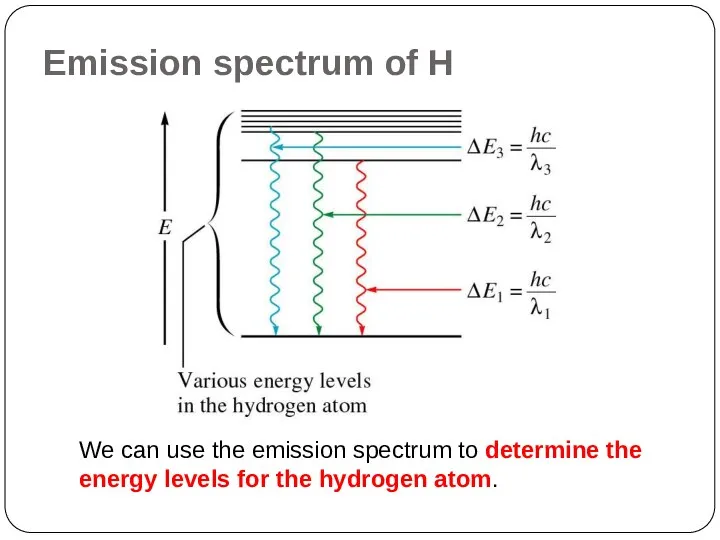





 Презентация Формирование универсальных учебных действий при изучении темы Давление твёрдых тел. жидкостей и газов
Презентация Формирование универсальных учебных действий при изучении темы Давление твёрдых тел. жидкостей и газов Что такое электричество?
Что такое электричество? Абсолютно твёрдое тело
Абсолютно твёрдое тело Двигатель Mercedes 2. Топливная система
Двигатель Mercedes 2. Топливная система Глава 3. Работа и энергия. Тема §1. Энергия, работа, мощность
Глава 3. Работа и энергия. Тема §1. Энергия, работа, мощность Волновые явления вблизи границы раздела сред. Плоские ЭВМ в неограниченных средах. Лекция 9
Волновые явления вблизи границы раздела сред. Плоские ЭВМ в неограниченных средах. Лекция 9 Тепловое излучение. Глава 5
Тепловое излучение. Глава 5 Материаловедение
Материаловедение Автоматизация управления в системах отопления
Автоматизация управления в системах отопления Устройство и принцип действия холодильника
Устройство и принцип действия холодильника Источники и приемники оптического излучения
Источники и приемники оптического излучения Электроемкость. Конденсаторы. Энергия заряженного конденсатора
Электроемкость. Конденсаторы. Энергия заряженного конденсатора Голография
Голография Элементы теории атомного ядра
Элементы теории атомного ядра Деятельностный подход в преподавании физики, как средство повышения качества знаний учащихся
Деятельностный подход в преподавании физики, как средство повышения качества знаний учащихся Обобщающий урок-игра по физике, 9 класс
Обобщающий урок-игра по физике, 9 класс План трассы. Вписывание круговых кривых с переходными кривыми
План трассы. Вписывание круговых кривых с переходными кривыми Stress analysis versus modes of fracture in composites
Stress analysis versus modes of fracture in composites Типы оптических спектров. Спектральный анализ
Типы оптических спектров. Спектральный анализ Инфрақызыл и раман спектроскопия
Инфрақызыл и раман спектроскопия През.ЮА.МС11-21
През.ЮА.МС11-21 Сравнительная характеристика постоянного электрического и постоянного магнитного поля
Сравнительная характеристика постоянного электрического и постоянного магнитного поля Теплотехника. Энтропия и первое начало термодинамики. (Лекция 4)
Теплотехника. Энтропия и первое начало термодинамики. (Лекция 4) Измерение сопротивления и тангенса изоляции
Измерение сопротивления и тангенса изоляции Постоянный электрический ток. Решение задач повышенной сложности по физике
Постоянный электрический ток. Решение задач повышенной сложности по физике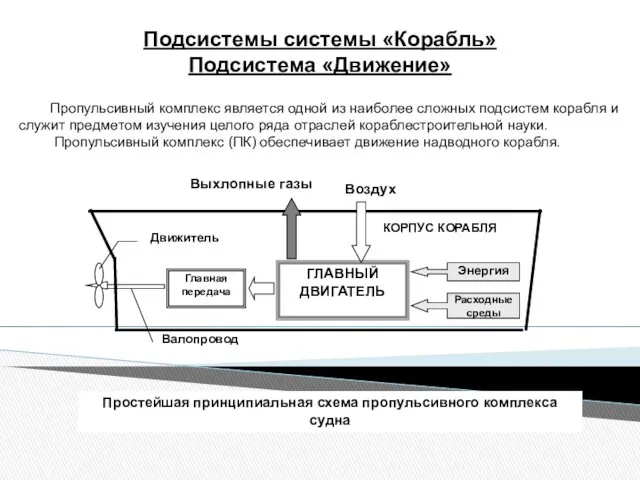 Подсистемы системы Корабль. Подсистема Движение
Подсистемы системы Корабль. Подсистема Движение Определение частоты вращения и крутящих моментов на всех валах привода и подбор электродвигателя
Определение частоты вращения и крутящих моментов на всех валах привода и подбор электродвигателя Новая тория прочности - механика разрушения
Новая тория прочности - механика разрушения