Содержание
- 2. Outline Thermal Energy Chemical Energy Electrolysis PV and electrolysis Fuel Cells
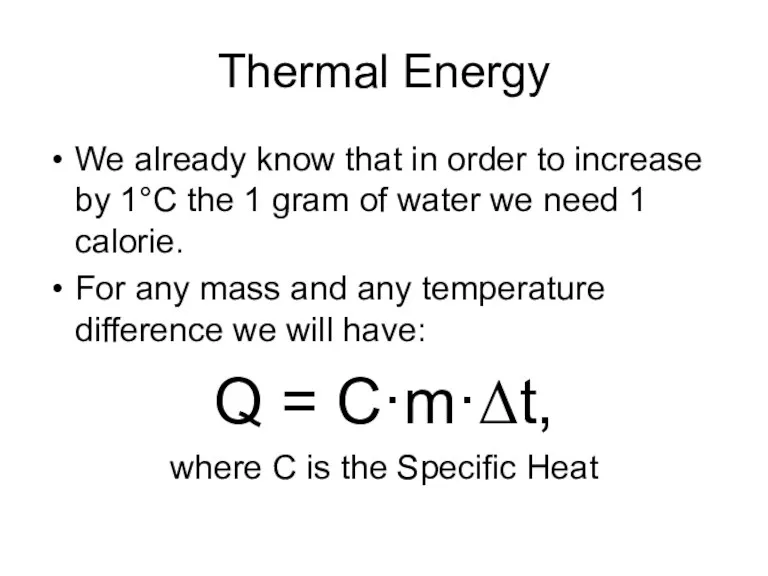
- 3. Thermal Energy We already know that in order to increase by 1°C the 1 gram of
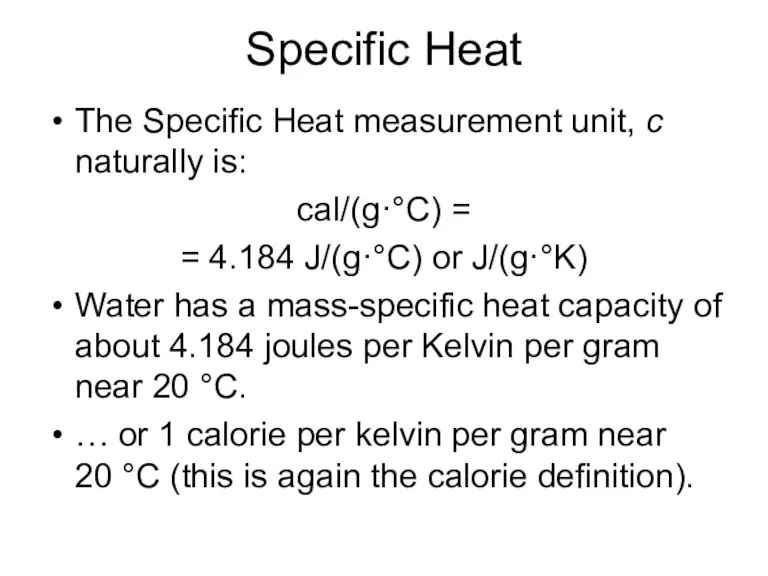
- 4. Specific Heat The Specific Heat measurement unit, c naturally is: cal/(g·°C) = = 4.184 J/(g·°C) or
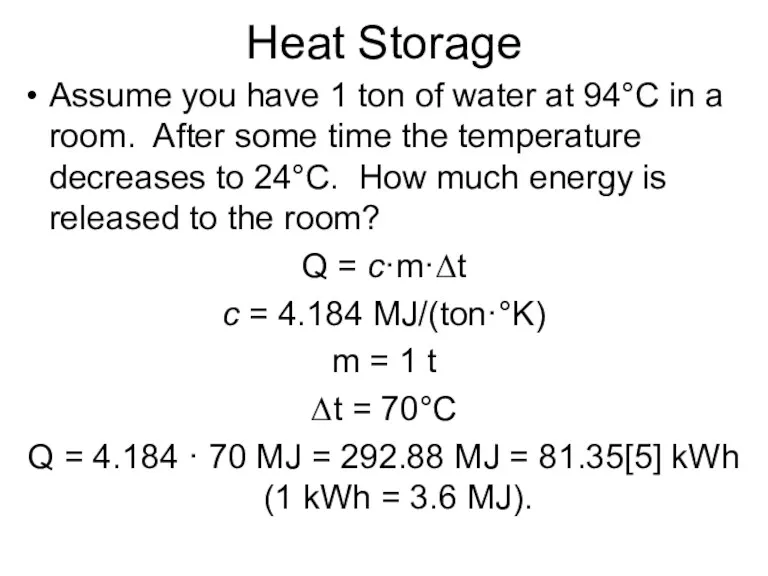
- 5. Heat Storage Assume you have 1 ton of water at 94°C in a room. After some
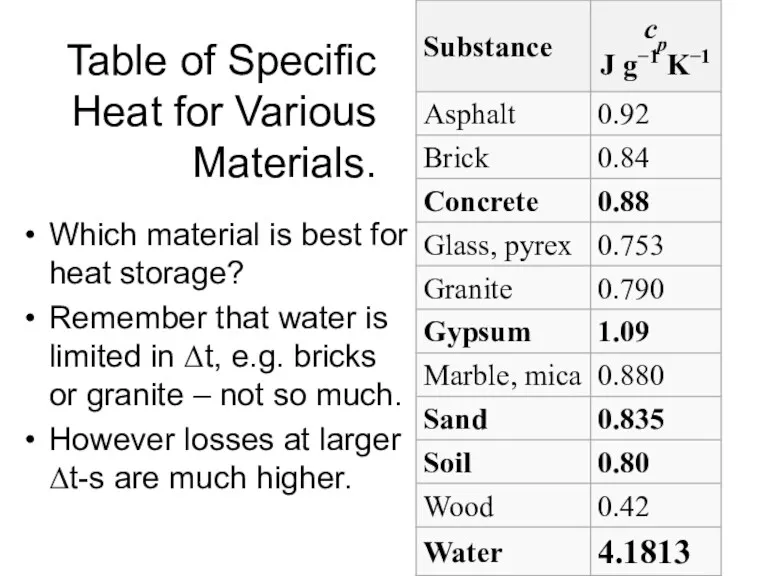
- 6. Table of Specific Heat for Various Materials. Which material is best for heat storage? Remember that
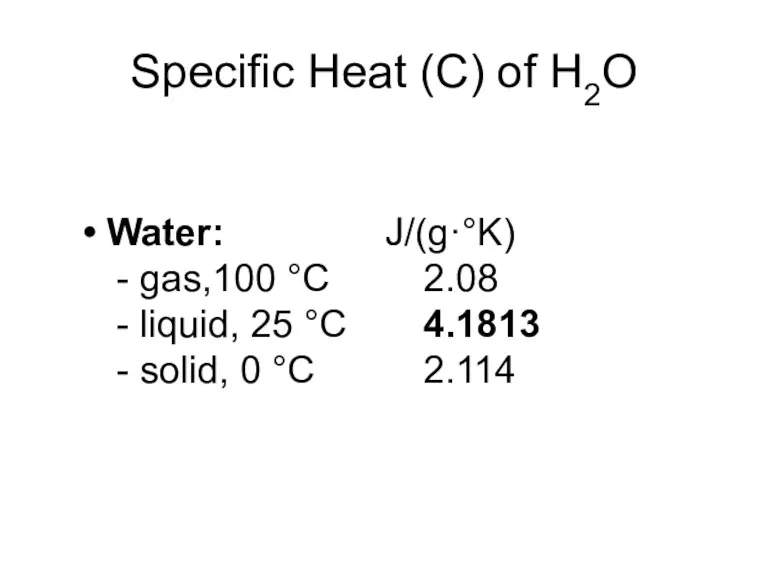
- 7. Specific Heat (C) of H2O Water: J/(g·°K) - gas,100 °C 2.08 - liquid, 25 °C 4.1813
- 8. Specific Heat
- 9. Losses Losses are linearly related to the temperature difference Δt (temperature gradient)!
- 10. Specific Heat of: Fusion and Vaporization Specific Heat of Fusion: Amount of energy needed to turn
- 11. H2O: From Ice to Vapor How much Energy is needed to turn ice into vapor? 5
- 12. H2O: From Ice to Vapor Energy needed to melt the ice: 333 J/g = specific heat
- 13. Phase change storage!
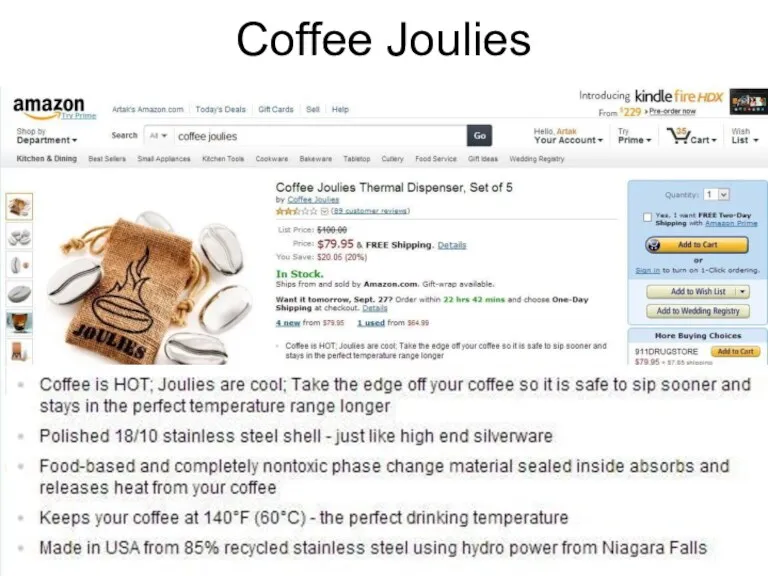
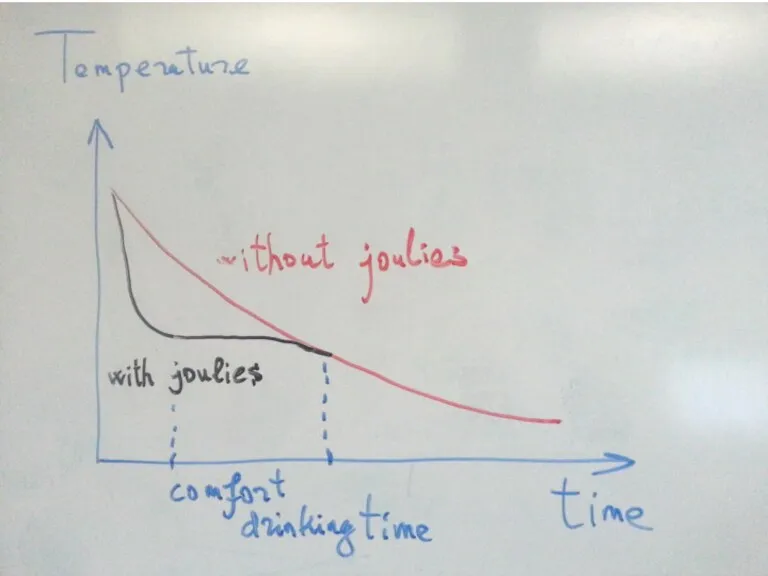
- 14. Coffee Joulies
- 15. Coffee Joulies
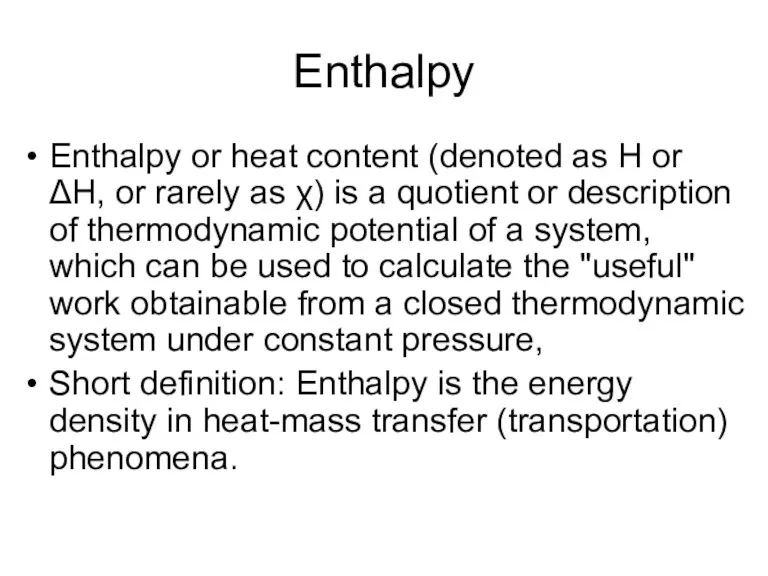
- 17. Enthalpy Enthalpy or heat content (denoted as H or ΔH, or rarely as χ) is a
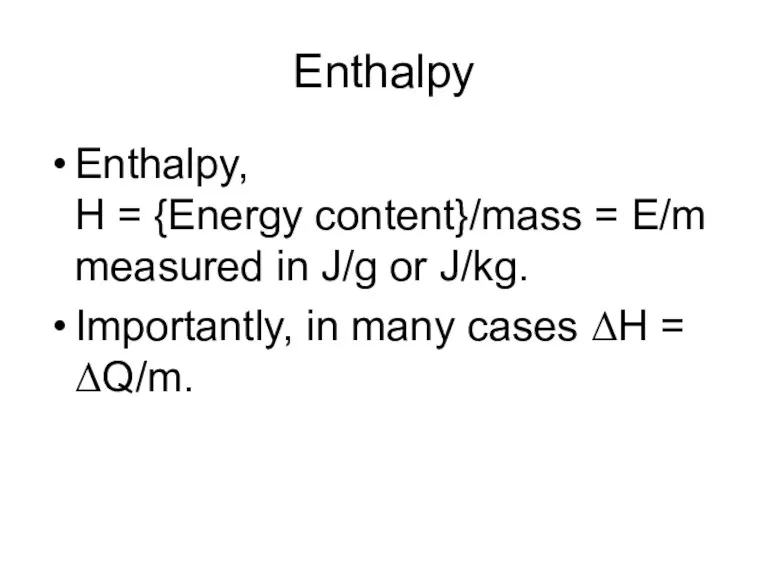
- 18. Enthalpy Enthalpy, H = {Energy content}/mass = E/m measured in J/g or J/kg. Importantly, in many
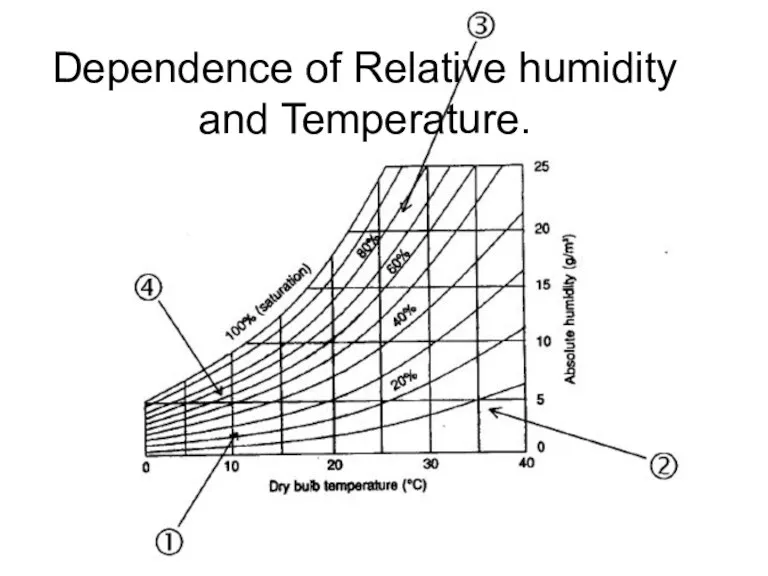
- 19. Humidity Absolute Relative Absolute Humidity = weight of water in the volume of air, g/m3; …
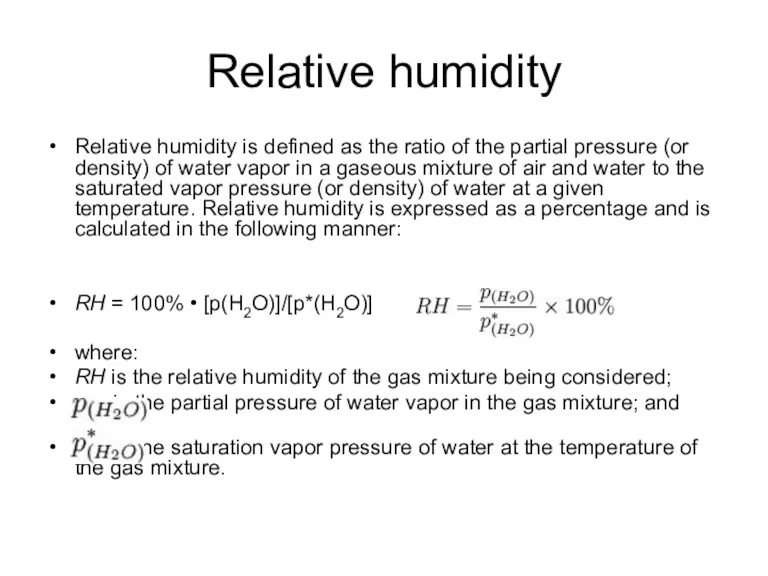
- 20. Relative humidity Relative humidity is defined as the ratio of the partial pressure (or density) of
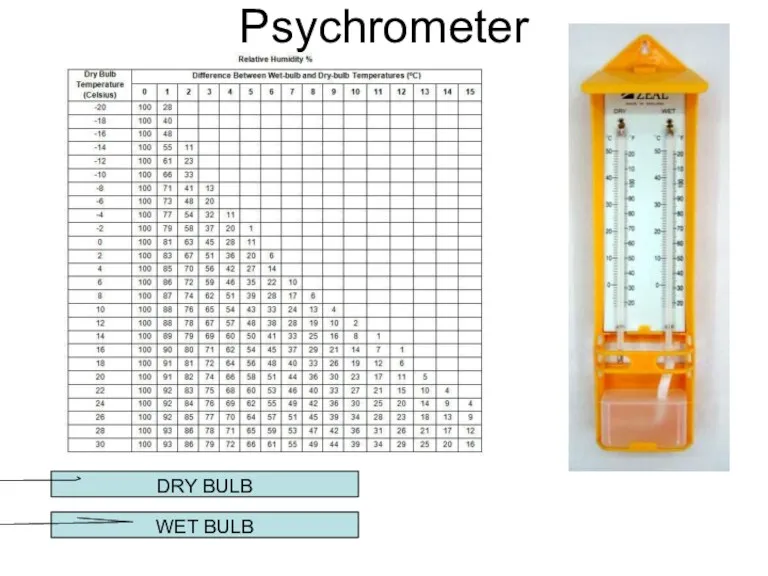
- 21. Psychrometer DRY BULB WET BULB
- 22. Dependence of Relative humidity and Temperature.
- 23. Anti-condensation bathroom mirror
- 24. Anti-condensation bathroom mirror
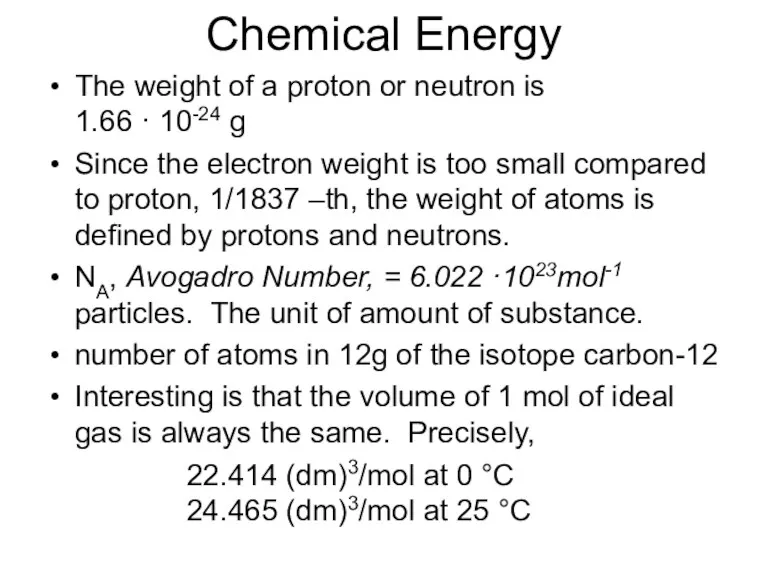
- 25. Chemical Energy The weight of a proton or neutron is 1.66 · 10-24 g Since the
- 26. Avogadro Number’s Holiday October 23 is called Mole Day. It is an informal holiday in honor
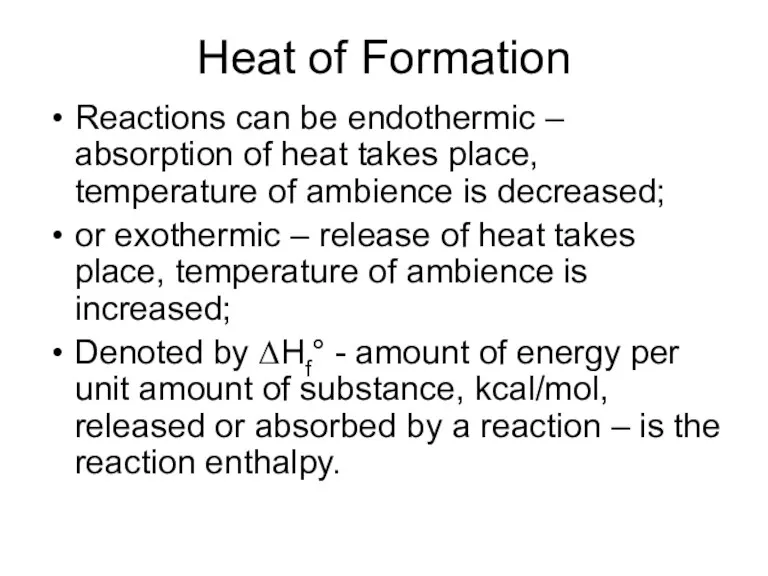
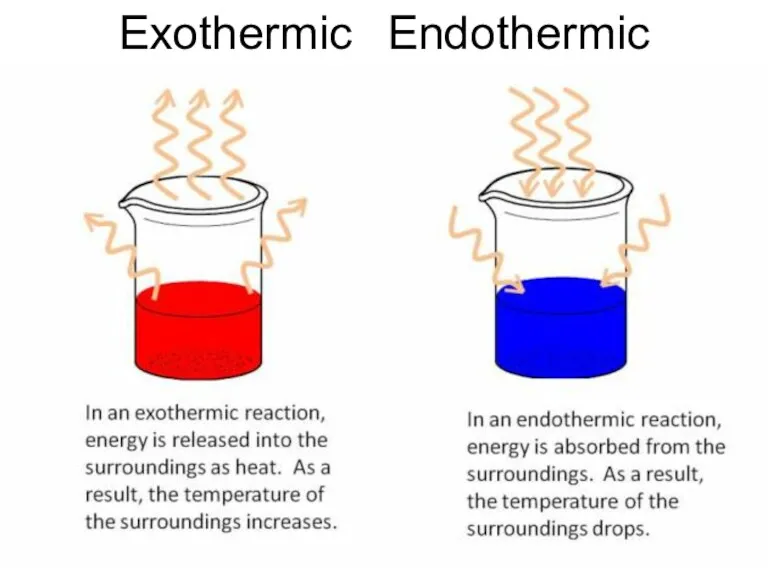
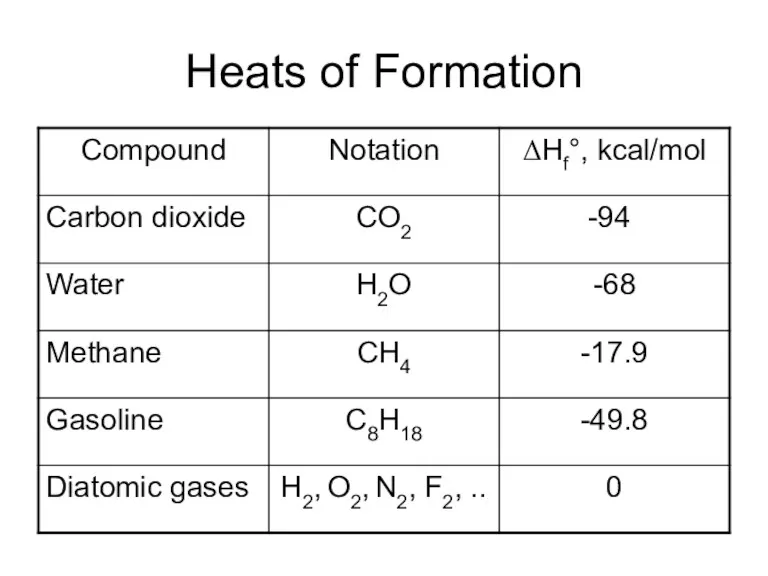
- 27. Heat of Formation Reactions can be endothermic – absorption of heat takes place, temperature of ambience
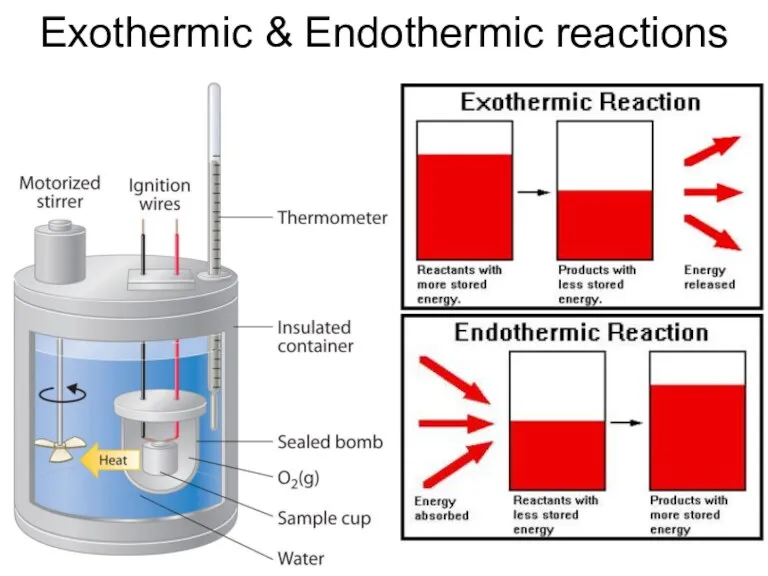
- 28. Exothermic Endothermic
- 29. Exothermic & Endothermic reactions
- 30. Heats of Formation
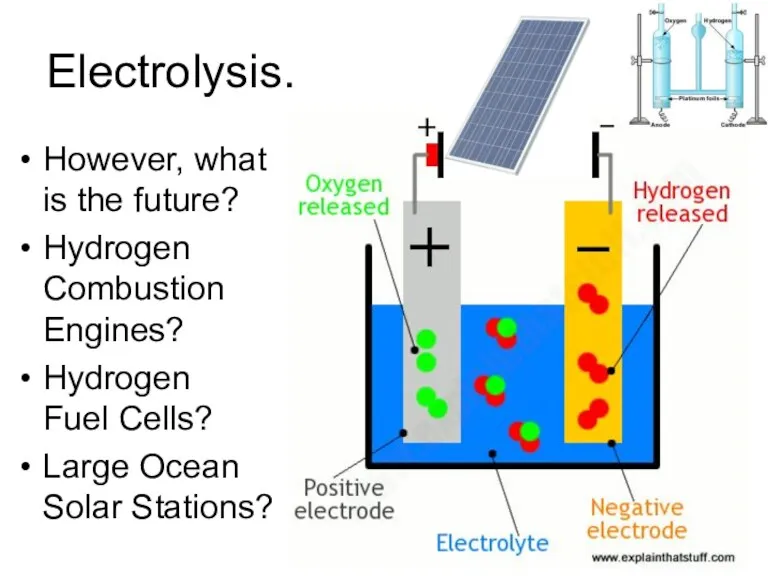
- 31. Hydrogen and water We all know: H2 + O2 ? H2O But the correct reaction formula
- 32. Electrolysis. However, what is the future? Hydrogen Combustion Engines? Hydrogen Fuel Cells? Large Ocean Solar Stations?
- 33. PV and electrolysis. Storage of solar energy is a problem yet to be solved. Hydrogen is
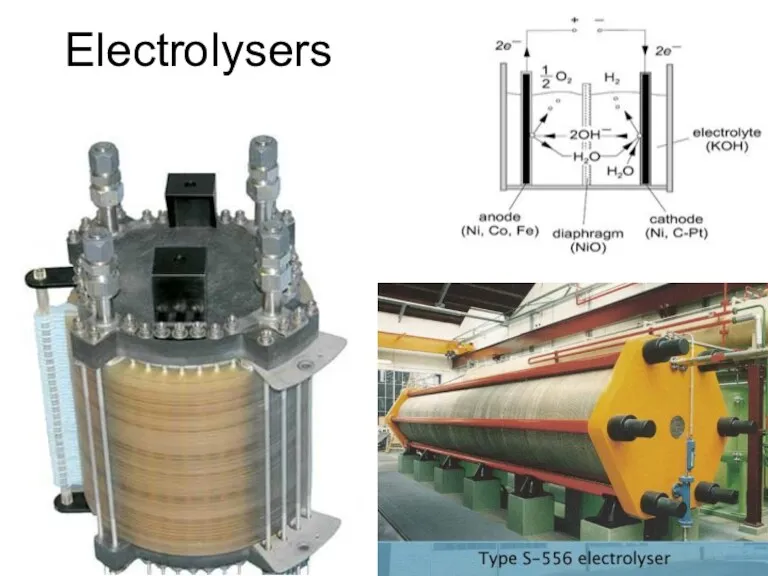
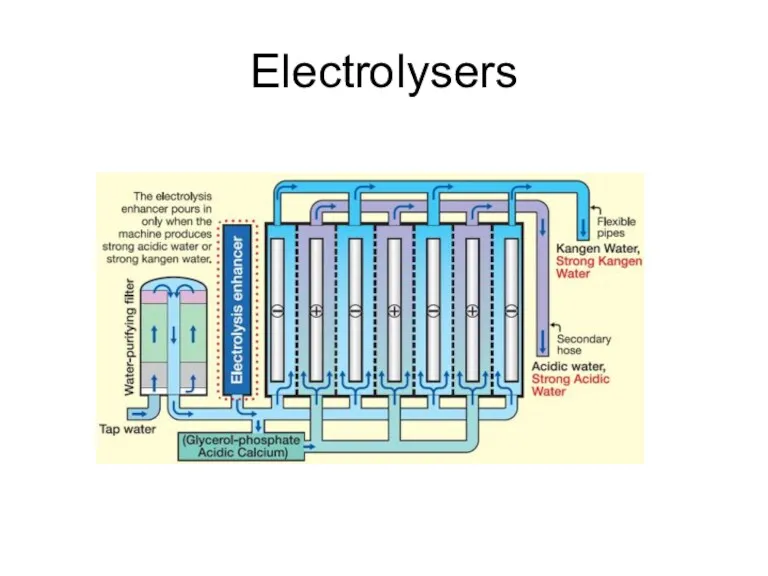
- 34. Electrolysers
- 35. Electrolysers
- 36. Electrolysers
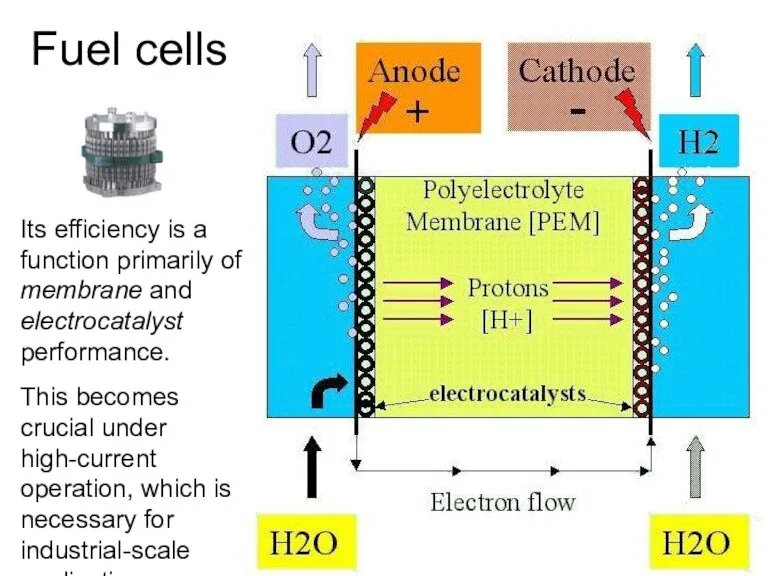
- 38. Its efficiency is a function primarily of membrane and electrocatalyst performance. This becomes crucial under high-current
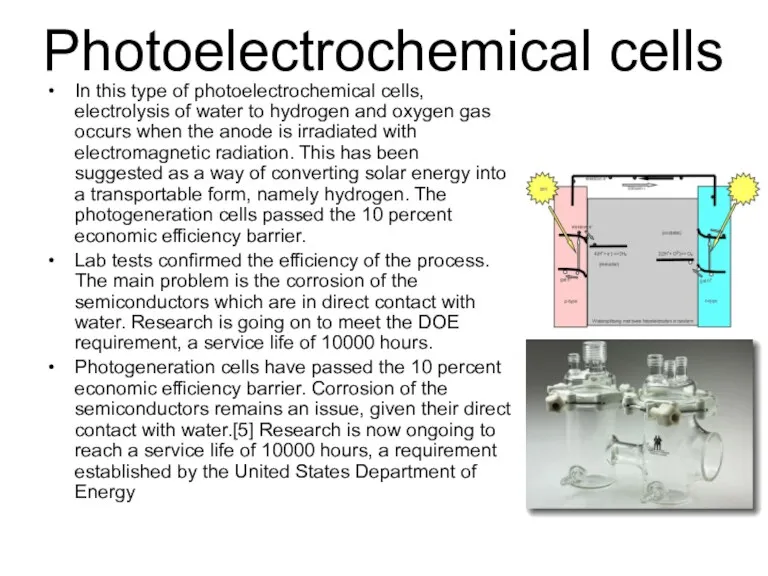
- 39. Photoelectrochemical cells In this type of photoelectrochemical cells, electrolysis of water to hydrogen and oxygen gas
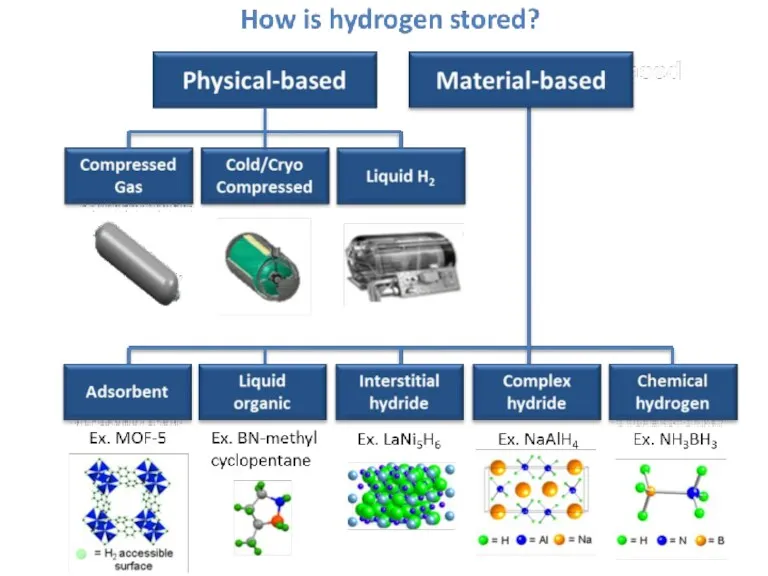
- 40. How to store Hydrogen? Cylinders – compressed hydrogen Metal Hydrate Compounds Cryogenic storage Chemical Storage Carbon
- 42. Cylinders – compressed hydrogen requires energy to acomplish lower energy density when compared to a traditional
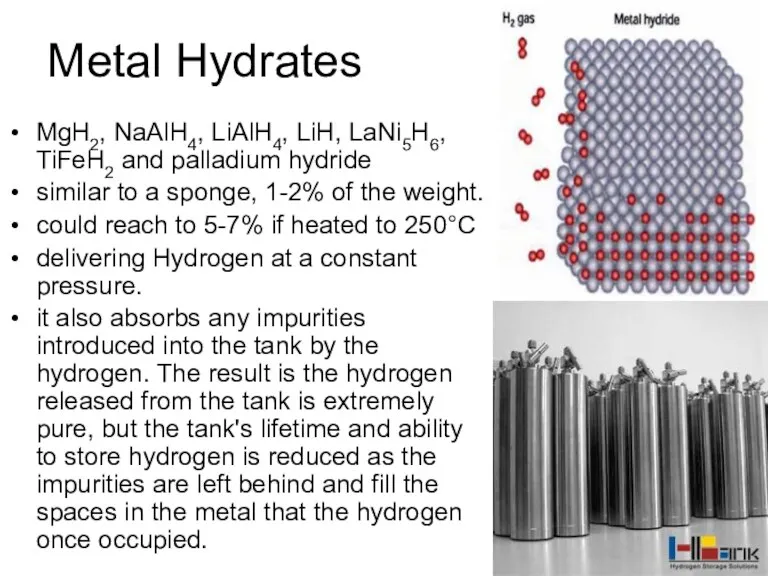
- 43. Metal Hydrates MgH2, NaAlH4, LiAlH4, LiH, LaNi5H6, TiFeH2 and palladium hydride similar to a sponge, 1-2%
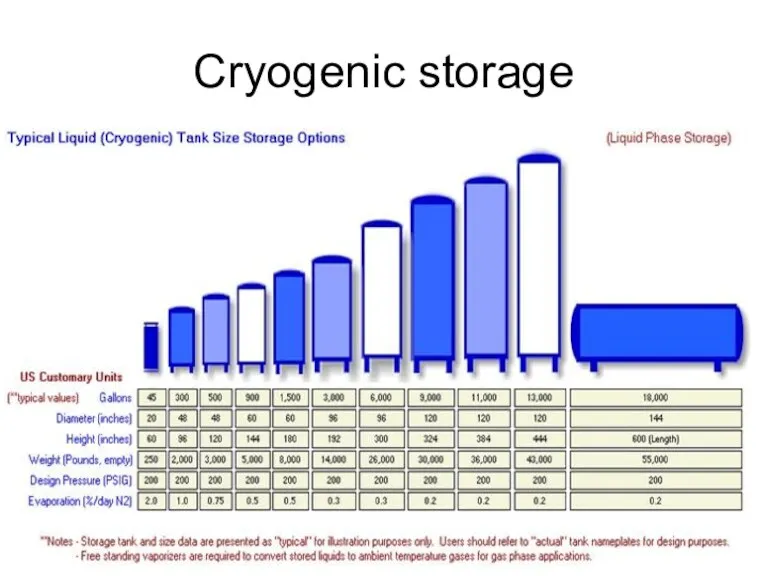
- 44. Cryogenic storage Liquid hydrogen typically has to be stored at 20o Kelvin or -253o C. again,
- 45. Cryogenic storage
- 46. Chemical Storage Some examples of various techniques include ammonia cracking, partial oxidation, methanol cracking, etc. These
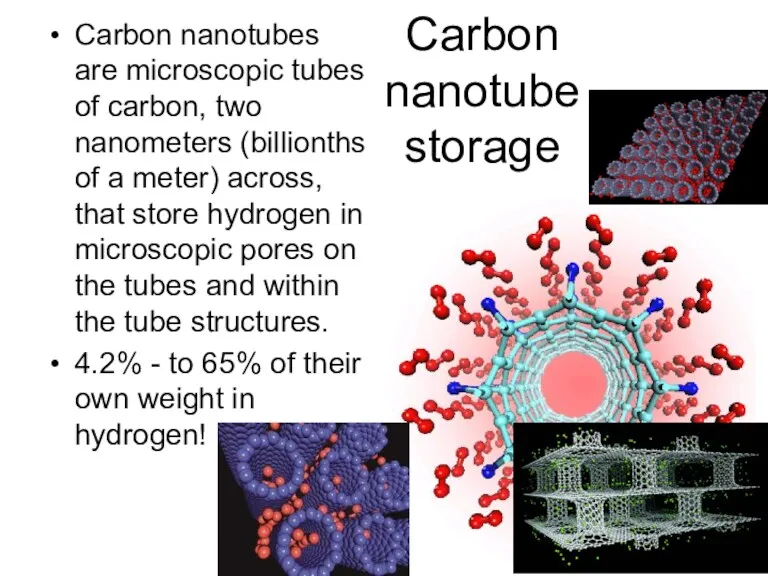
- 47. Carbon nanotube storage Carbon nanotubes are microscopic tubes of carbon, two nanometers (billionths of a meter)
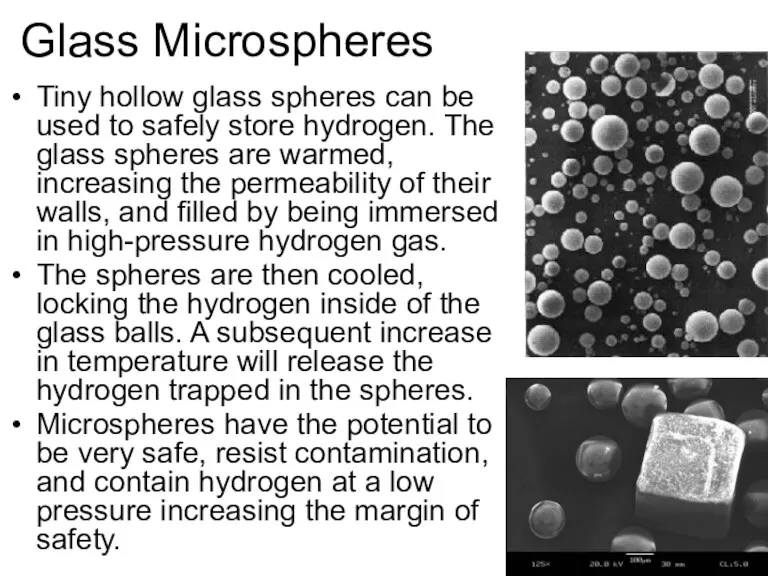
- 48. Glass Microspheres Tiny hollow glass spheres can be used to safely store hydrogen. The glass spheres
- 49. Liquid Carrier (Carbohydrate) Storage This is the technical term for the hydrogen being stored in the
- 50. Hydrogen Safety The range of explosion proportion in air is rather wide, starting at 4%. Hydrogen
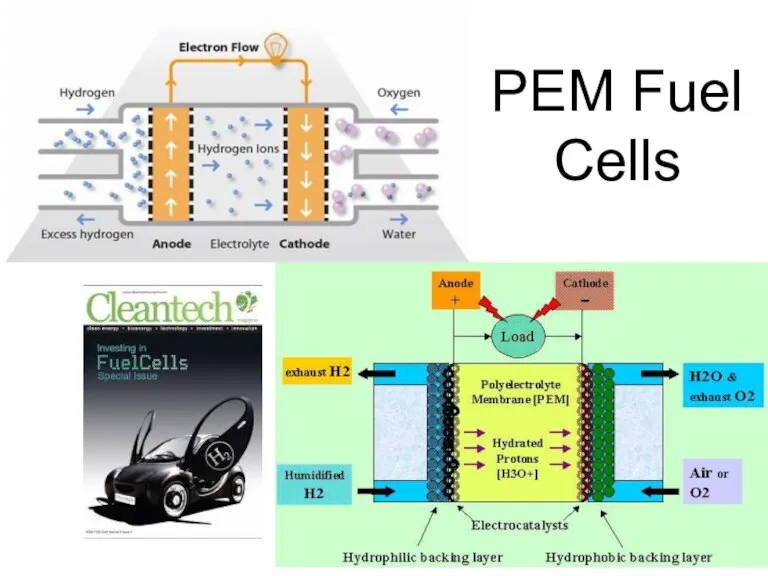
- 51. Hydrogen Use Internal Combustion Engines PEM Fuel Cells
- 52. PEM Fuel Cells
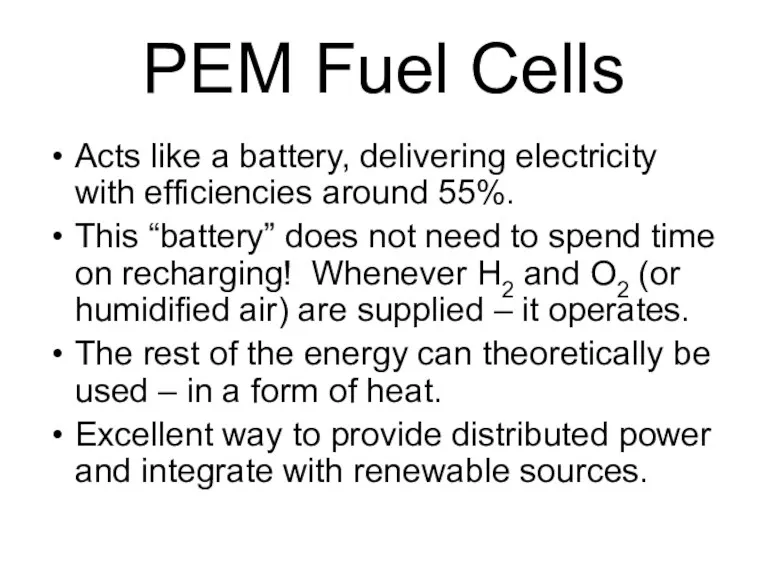
- 53. PEM Fuel Cells Acts like a battery, delivering electricity with efficiencies around 55%. This “battery” does
- 54. PEM Fuel Cells
- 55. PEM Fuel Cells H2ECOnomy
- 57. Скачать презентацию























 Исследование последовательного соединения проводников
Исследование последовательного соединения проводников Динамика материальной точки. Лекция 2
Динамика материальной точки. Лекция 2 Подготовка к ОГЭ 2021 году в новом формате, по физике
Подготовка к ОГЭ 2021 году в новом формате, по физике Механические характеристики материалов
Механические характеристики материалов Синтез наноматериалов золь-гель методом. (Лекция 5)
Синтез наноматериалов золь-гель методом. (Лекция 5) Устройство и назначение передач винт-гайка
Устройство и назначение передач винт-гайка Излучение и прием электромагнитных волн. Принципы радиосвязи
Излучение и прием электромагнитных волн. Принципы радиосвязи Волновые явления
Волновые явления Методы моментов. Метод сферических гармоник. Уравнение переноса в Р1-приближении. Диффузионное приближение
Методы моментов. Метод сферических гармоник. Уравнение переноса в Р1-приближении. Диффузионное приближение Механические подвески автомобиля
Механические подвески автомобиля Особенности заданий ЕГЭ. Колебания и волны
Особенности заданий ЕГЭ. Колебания и волны Дифракция құбылысы. Френел және Фраунгофер жуықтаулары. Амплитудалық және фазалық дифракциялық торлар
Дифракция құбылысы. Френел және Фраунгофер жуықтаулары. Амплитудалық және фазалық дифракциялық торлар презентация Обнаружение магнитного поля по его действию на электрический ток
презентация Обнаружение магнитного поля по его действию на электрический ток Волновая оптика
Волновая оптика Изобретение радио. Принципы радиосвязи. Телевидение
Изобретение радио. Принципы радиосвязи. Телевидение Електричний заряд. Закон збереження електричного заряду
Електричний заряд. Закон збереження електричного заряду Аэродинамический нагрев
Аэродинамический нагрев Сила трения. Автор Максимова Наталья Сергеевна
Сила трения. Автор Максимова Наталья Сергеевна Ходовая часть боевой машины пехоты. Тема 13
Ходовая часть боевой машины пехоты. Тема 13 испарение и конденсация
испарение и конденсация Резьбовые соединения
Резьбовые соединения Молекулярная физика и термодинамика
Молекулярная физика и термодинамика Конспект урока на тему: Плотность вещества 7 класс
Конспект урока на тему: Плотность вещества 7 класс Обертальний рух. Приклади обертального руху в природі
Обертальний рух. Приклади обертального руху в природі Электрооборудование автомобилей. Сигнальное оборудование. (Урок 8)
Электрооборудование автомобилей. Сигнальное оборудование. (Урок 8) Простые механизмы. Рычаг. Равновесие сил на рычаге
Простые механизмы. Рычаг. Равновесие сил на рычаге Плотность вещества
Плотность вещества Учебная практика (техническое обслуживание автомобилей)
Учебная практика (техническое обслуживание автомобилей)