Содержание
- 2. BOND Hydrocarbons that contain the carbon-carbon triple bond –C (triple)C- are called alkynes. Each triple bond
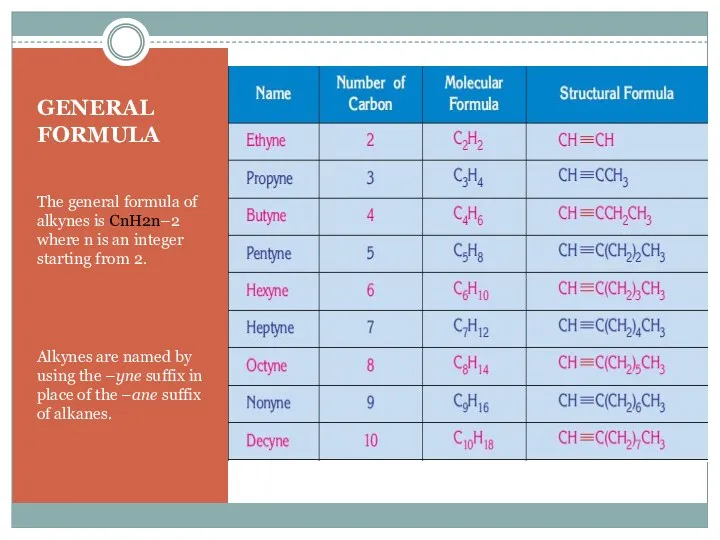
- 3. GENERAL FORMULA The general formula of alkynes is CnH2n–2 where n is an integer starting from
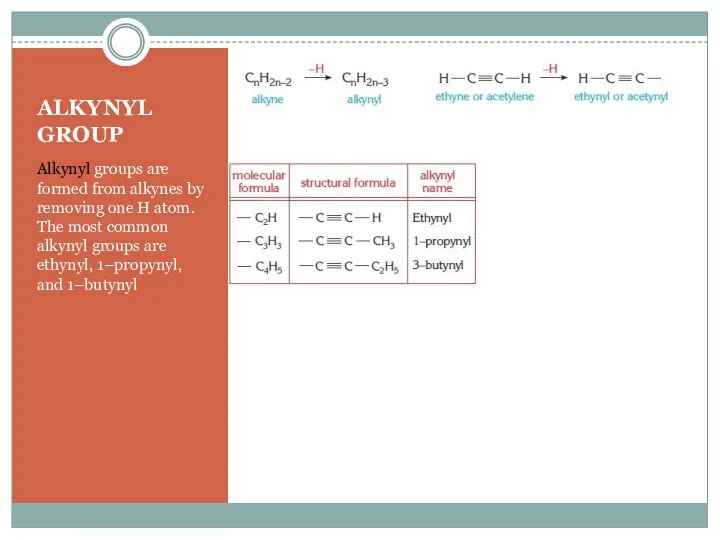
- 4. ALKYNYL GROUP Alkynyl groups are formed from alkynes by removing one H atom. The most common
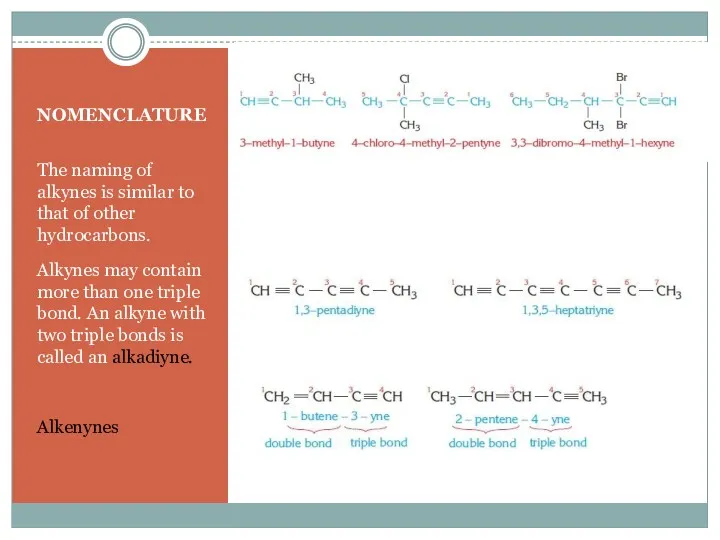
- 5. NOMENCLATURE The naming of alkynes is similar to that of other hydrocarbons. Alkynes may contain more
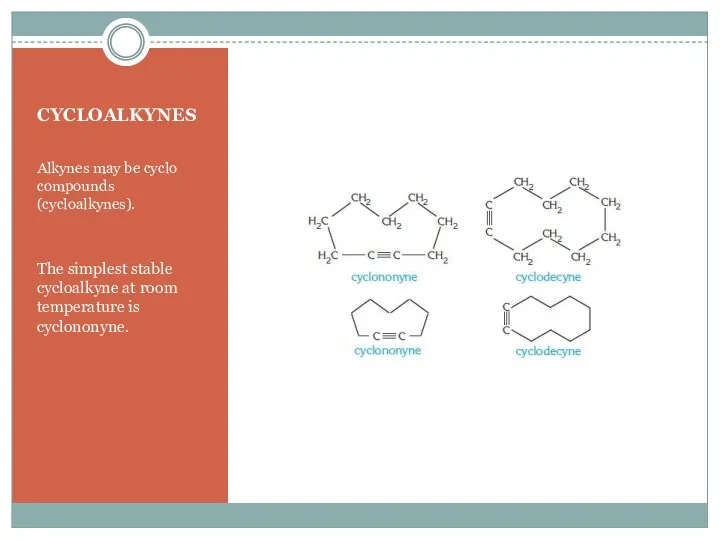
- 6. CYCLOALKYNES Alkynes may be cyclo compounds (cycloalkynes). The simplest stable cycloalkyne at room temperature is cyclononyne.
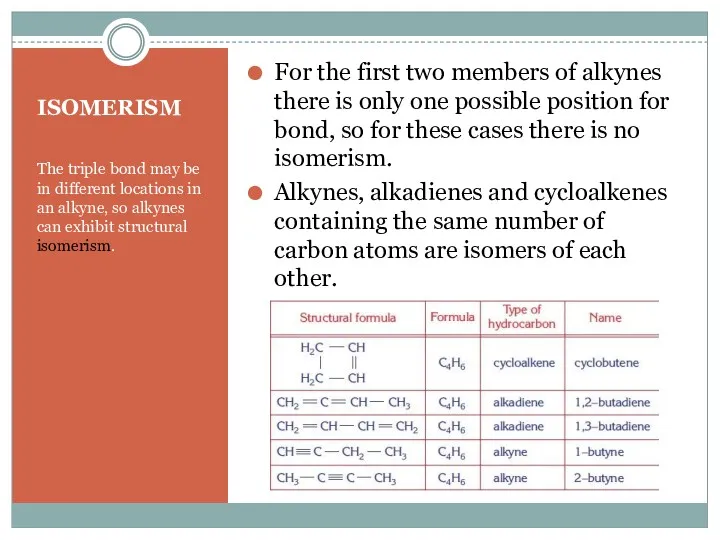
- 7. ISOMERISM The triple bond may be in different locations in an alkyne, so alkynes can exhibit
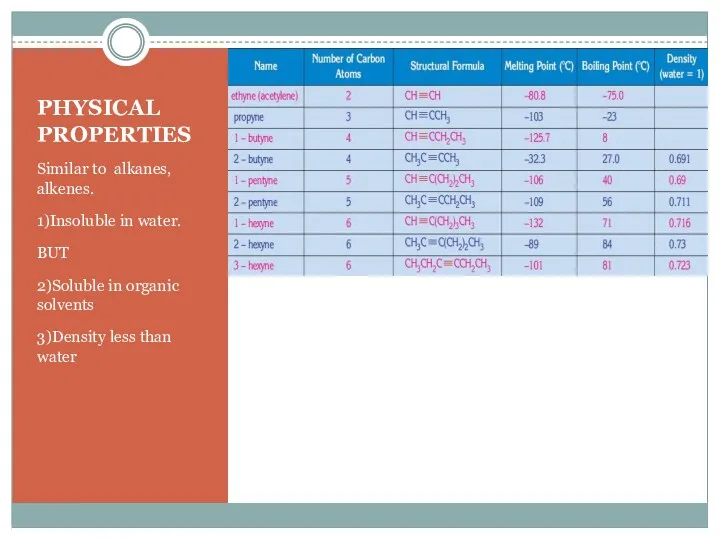
- 8. PHYSICAL PROPERTIES Similar to alkanes, alkenes. 1)Insoluble in water. BUT 2)Soluble in organic solvents 3)Density less
- 9. CHEMICAL PROPERTIES Alkynes are unsaturated compounds and their chemical properties are similar to alkenes. Alkynes undergo
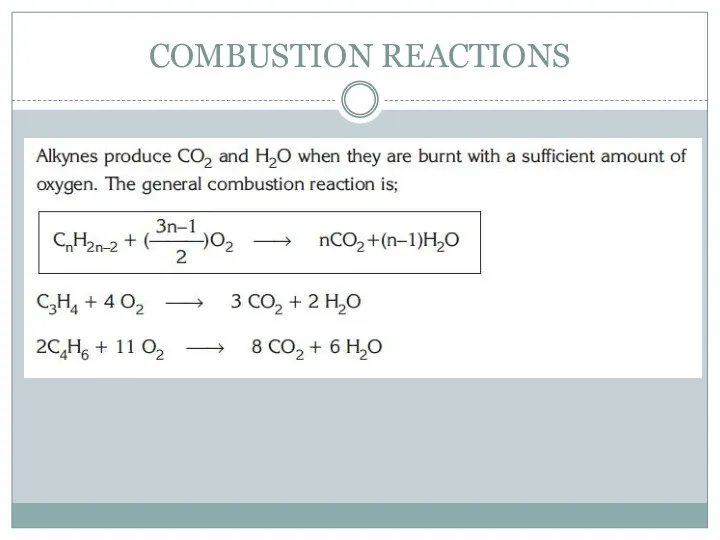
- 10. COMBUSTION REACTIONS
- 11. ADDITION REACTIONS Addition reactions occur by breaking the π bonds of the triple bond. Hydrogen, halogens,
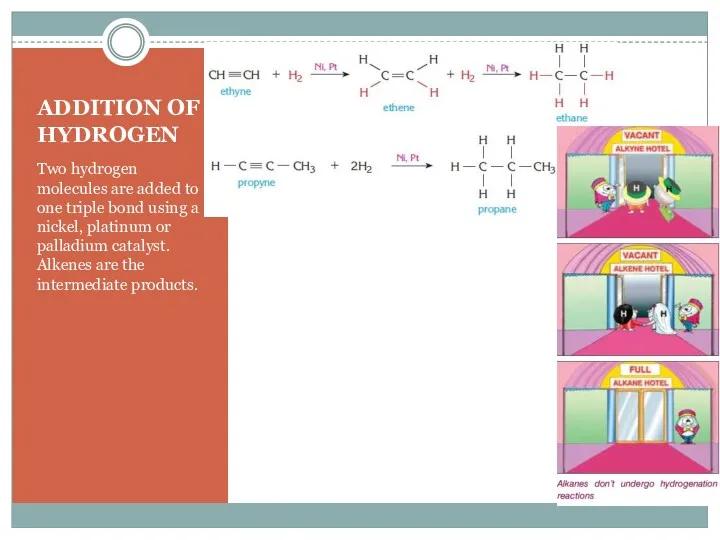
- 12. ADDITION OF HYDROGEN Two hydrogen molecules are added to one triple bond using a nickel, platinum
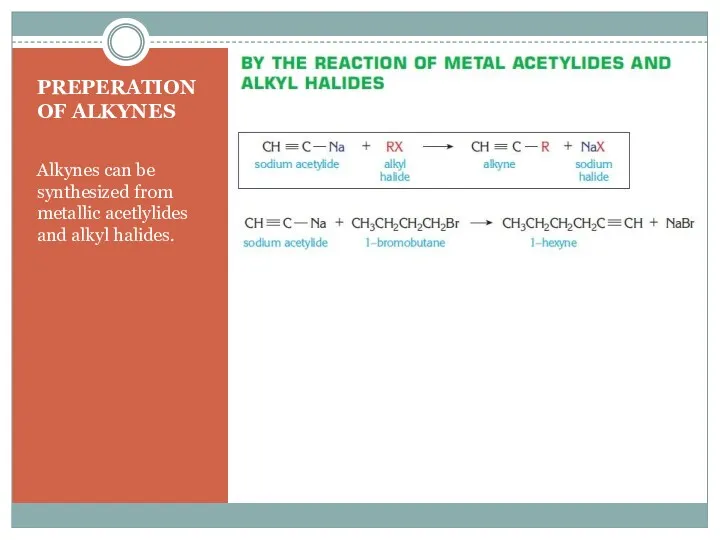
- 13. PREPERATION OF ALKYNES Alkynes can be synthesized from metallic acetlylides and alkyl halides.
- 14. ACETYLENE Acetylene, the first member of the alkyne series, is one of the major chemicals used
- 15. USES OF ALKYNES Histrionicotoxin toxic alkyne present in South American frogs used to make poison-tipped arrows
- 17. Скачать презентацию




 Химия в жизни общества
Химия в жизни общества Organic molecules
Organic molecules Первичная переработка нефти
Первичная переработка нефти Состав газированной воды
Состав газированной воды Железо. Характеристика химического элемента железа по его положению в ПСХЭ и строению атома
Железо. Характеристика химического элемента железа по его положению в ПСХЭ и строению атома Галогены (солеобразующие)
Галогены (солеобразующие) Склад та властивості основних класів неорганічних сполук
Склад та властивості основних класів неорганічних сполук Научно-исследовательская работа Вся правда о продуктах с сахарозаменителями
Научно-исследовательская работа Вся правда о продуктах с сахарозаменителями Катионы 1, 2 аналитических групп
Катионы 1, 2 аналитических групп Углекислый газ
Углекислый газ Неон. Получение
Неон. Получение Периодическая система Д. И. Менделеева
Периодическая система Д. И. Менделеева Тема 1. Обработка вооружения, техники и обмундирования. Дегазирующие, дезактивирующие и дезинфицирующие вещества и растворы
Тема 1. Обработка вооружения, техники и обмундирования. Дегазирующие, дезактивирующие и дезинфицирующие вещества и растворы
 Химическая связь
Химическая связь Углеводы Cn(H2O)m
Углеводы Cn(H2O)m Генетическая связь между классами органических веществ
Генетическая связь между классами органических веществ Типы химических реакций в органической химии
Типы химических реакций в органической химии СПЛАВЫ. КОРРОЗИЯ МЕТАЛЛОВ
СПЛАВЫ. КОРРОЗИЯ МЕТАЛЛОВ Карбоновые кислоты и их функциональные производные
Карбоновые кислоты и их функциональные производные Хімічні сполуки у побуті
Хімічні сполуки у побуті Основные классы неорганических веществ
Основные классы неорганических веществ Мир первозданной красоты. Природные уникумы Урала
Мир первозданной красоты. Природные уникумы Урала Химический элемент и вещество
Химический элемент и вещество Тема 1. Металлы и сплавы
Тема 1. Металлы и сплавы Влияние спирта на здоровье человека
Влияние спирта на здоровье человека Превращения веществ. Роль химии в жизни человека
Превращения веществ. Роль химии в жизни человека Топливо. Виды топлива. Химический состав топлива. Основные характеристики топлива. Марки топлива
Топливо. Виды топлива. Химический состав топлива. Основные характеристики топлива. Марки топлива