Содержание
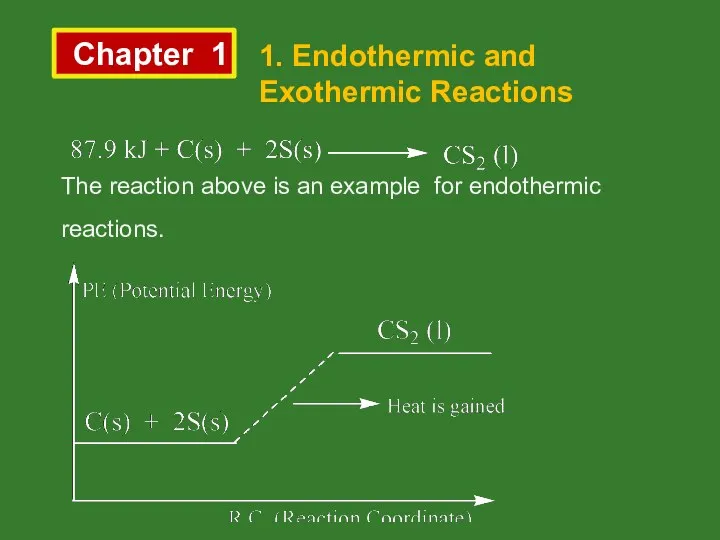
- 2. Chapter 1 1. Endothermic and Exothermic Reactions The reaction above is an example for endothermic reactions.
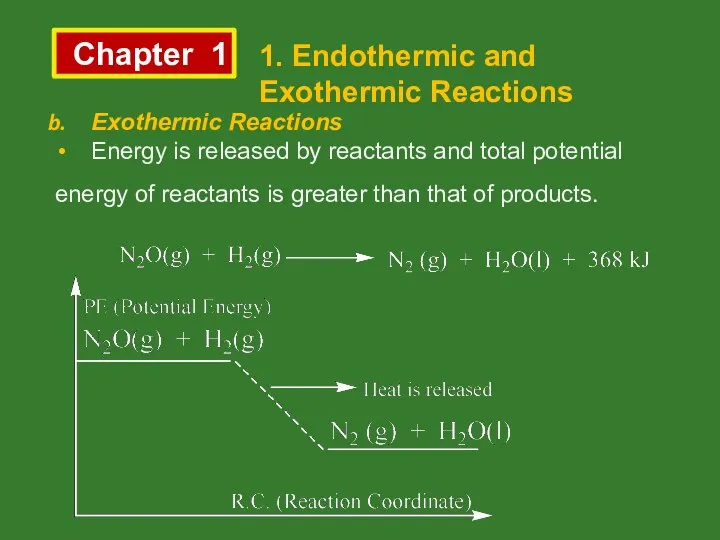
- 3. Chapter 1 1. Endothermic and Exothermic Reactions Exothermic Reactions Energy is released by reactants and total
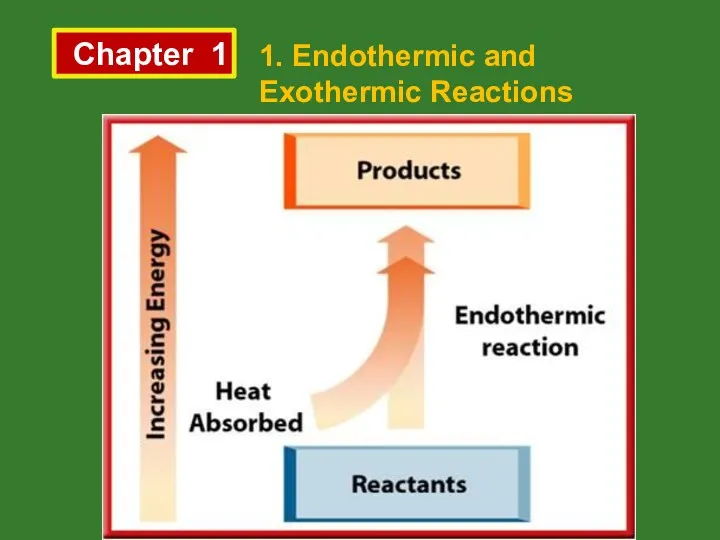
- 4. Chapter 1 1. Endothermic and Exothermic Reactions
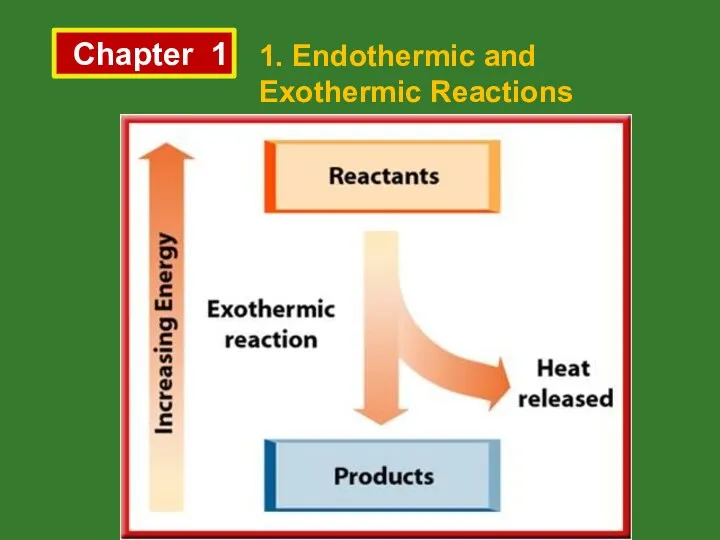
- 5. Chapter 1 1. Endothermic and Exothermic Reactions
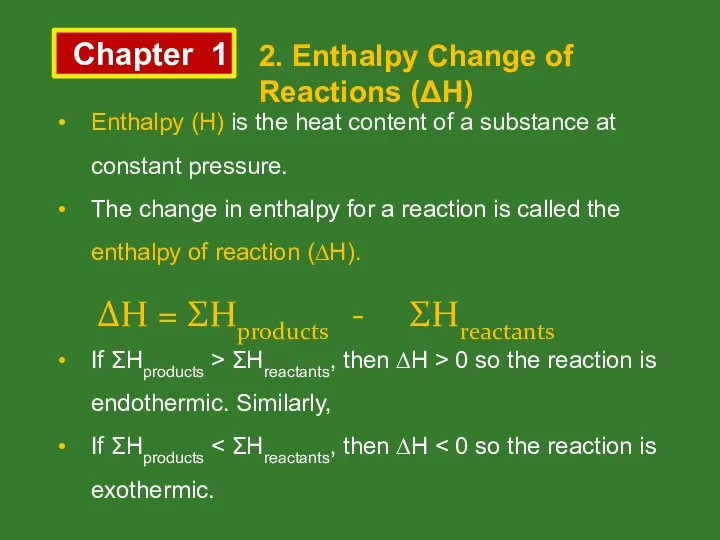
- 6. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Enthalpy (H) is the heat content of a
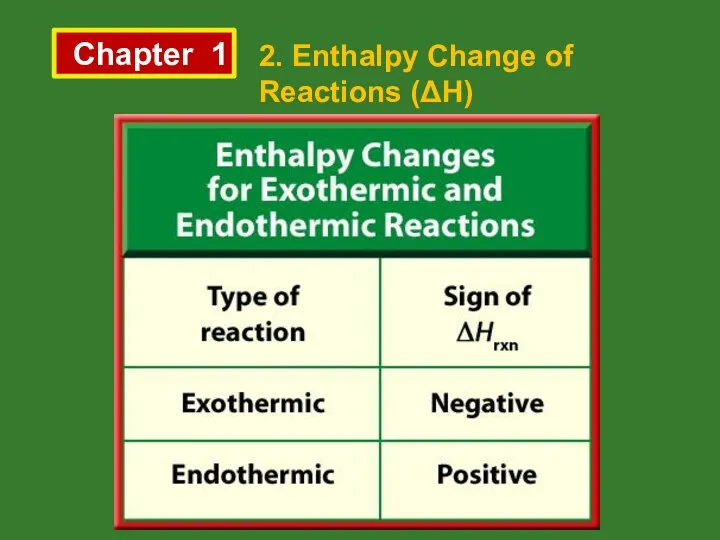
- 7. Chapter 1 2. Enthalpy Change of Reactions (ΔH)
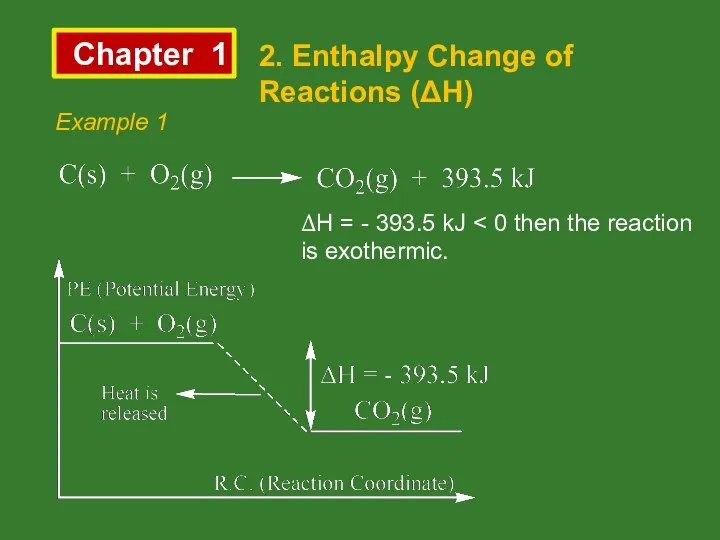
- 8. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Example 1 ΔH = - 393.5 kJ
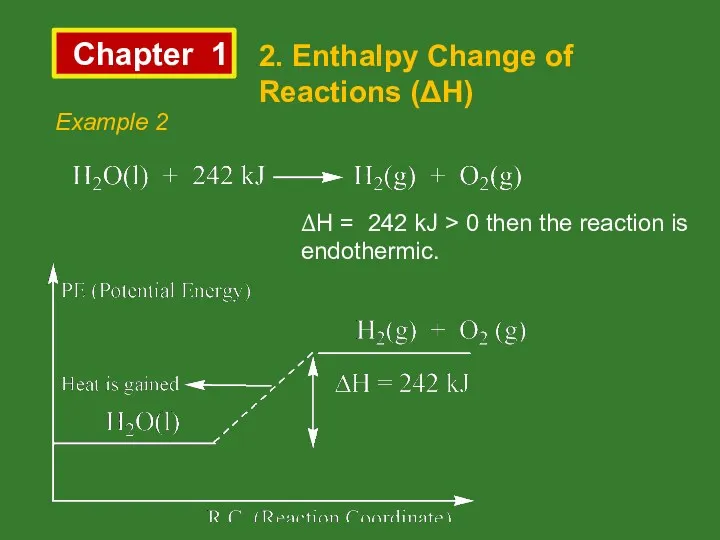
- 9. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Example 2 ΔH = 242 kJ > 0
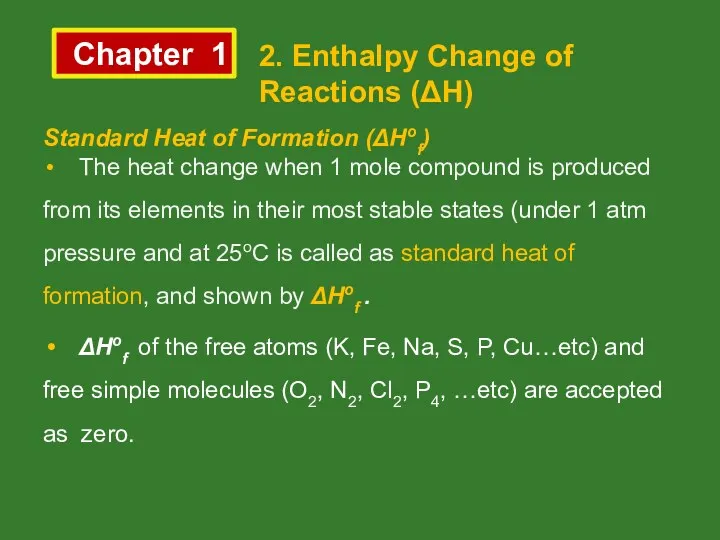
- 10. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Standard Heat of Formation (ΔHof) The heat change
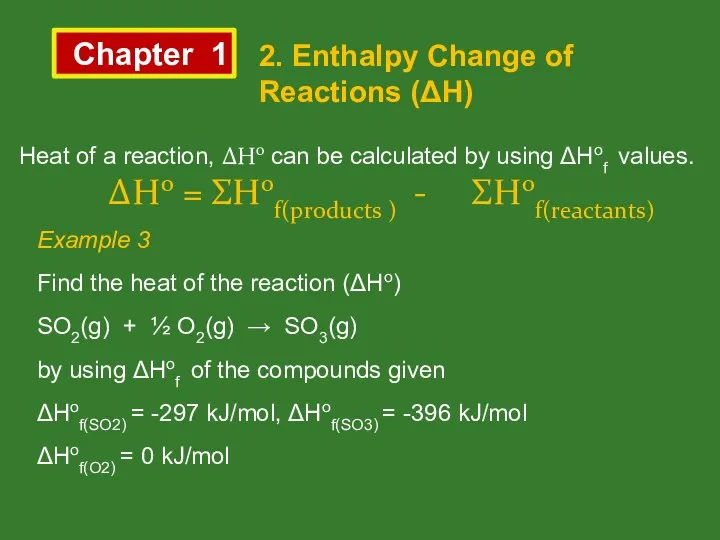
- 11. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Heat of a reaction, ΔHo can be calculated
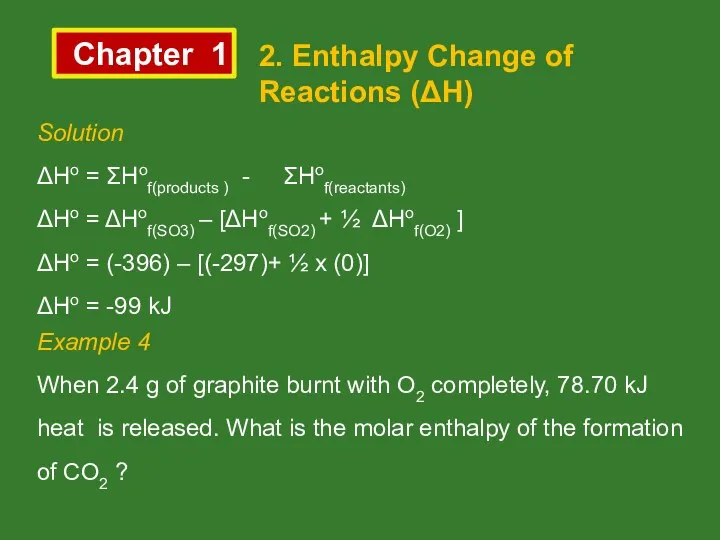
- 12. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Solution ΔHo = ΣHof(products ) - ΣHof(reactants) ΔHo
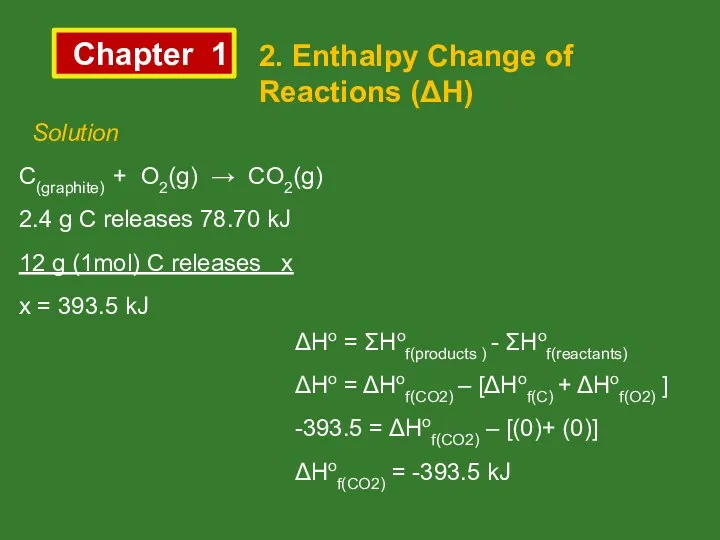
- 13. Chapter 1 2. Enthalpy Change of Reactions (ΔH) Solution C(graphite) + O2(g) → CO2(g) 2.4 g
- 15. Скачать презентацию
 Современная химия. (Лекция 6)
Современная химия. (Лекция 6) Кремний и его соединения для 11 класса
Кремний и его соединения для 11 класса Серебро. Нахождение в природе
Серебро. Нахождение в природе Получение каталитического слоя на основе углеродных нанотрубок с наночастицами платины для водородно–воздушных топливных элементов
Получение каталитического слоя на основе углеродных нанотрубок с наночастицами платины для водородно–воздушных топливных элементов Генетическая связь между классами неорганических соединений. Урок 1
Генетическая связь между классами неорганических соединений. Урок 1 Основные интермедиаты в органических реакциях. Часть 1
Основные интермедиаты в органических реакциях. Часть 1 Токсикологическая химия. Токсичность химических соединений
Токсикологическая химия. Токсичность химических соединений Ұшқыш уларды оқшаулауды теориялық негіздеу
Ұшқыш уларды оқшаулауды теориялық негіздеу Качественные реакции на органические вещества
Качественные реакции на органические вещества Взаимодействия кислорода с металлом
Взаимодействия кислорода с металлом Мінерали та гірські породи
Мінерали та гірські породи Нитросоединения
Нитросоединения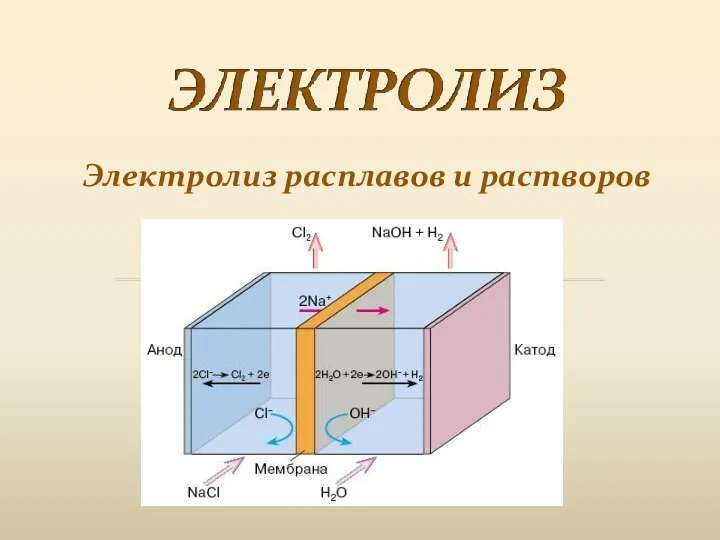 Электролиз расплавов и растворов
Электролиз расплавов и растворов Простые вещества металлы
Простые вещества металлы Свойства топлив. Марки бензинов и их характеристики
Свойства топлив. Марки бензинов и их характеристики Введение в геологию и минералогию
Введение в геологию и минералогию Кварц
Кварц Чистые вещества и смеси. Способы разделения смесей
Чистые вещества и смеси. Способы разделения смесей Красители и пигменты
Красители и пигменты Химиялық реакциялар. Амфотерлі негіздер
Химиялық реакциялар. Амфотерлі негіздер Подготовка учащихся к практическим турам олимпиад по химии
Подготовка учащихся к практическим турам олимпиад по химии Сложные эфиры. Жиры
Сложные эфиры. Жиры Химические свойства неорганических соединений. Лабораторная работа
Химические свойства неорганических соединений. Лабораторная работа Алкины. Самостоятельная работа
Алкины. Самостоятельная работа Электролиз водных растворов
Электролиз водных растворов Амины. Физические и химические свойства
Амины. Физические и химические свойства Ароматичні вуглеводні
Ароматичні вуглеводні Значение органической химии в жизни человека
Значение органической химии в жизни человека