Содержание
- 2. Atoms bond during chemical reactions to result in crystal formation. Crystals are defined as a solid
- 3. Types of chemical bonds in crystals 1.IONIC BONDS 2.COVALENT BONDS 3VAN DER WAALS BONDS 4.HYDROGEN BONDS
- 4. IONIC BONDS When ionic crystals are formed, electrons jump their orbits to bond with the corresponding
- 6. VAN DER WAALS BONDS A Van der Waals bond is a weak interaction between the atoms
- 10. Скачать презентацию
Слайд 2
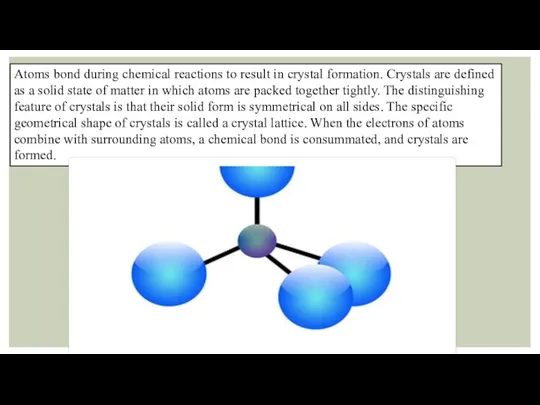
Atoms bond during chemical reactions to result in crystal formation. Crystals
Atoms bond during chemical reactions to result in crystal formation. Crystals
are defined as a solid state of matter in which atoms are packed together tightly. The distinguishing feature of crystals is that their solid form is symmetrical on all sides. The specific geometrical shape of crystals is called a crystal lattice. When the electrons of atoms combine with surrounding atoms, a chemical bond is consummated, and crystals are formed.
Слайд 3
Types of chemical bonds in crystals
1.IONIC BONDS
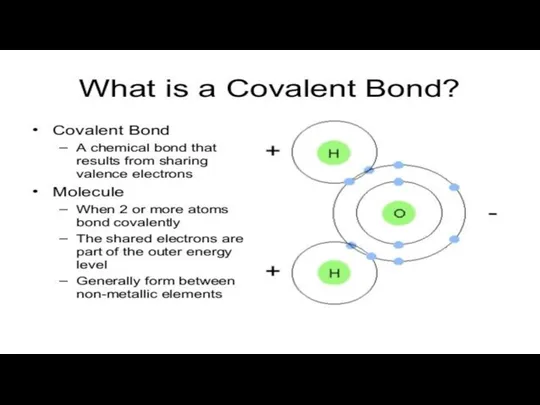
2.COVALENT BONDS
3VAN DER WAALS BONDS
4.HYDROGEN
Types of chemical bonds in crystals
1.IONIC BONDS
2.COVALENT BONDS
3VAN DER WAALS BONDS
4.HYDROGEN
BONDS
5.METALLIC BONDS
5.METALLIC BONDS
Слайд 4
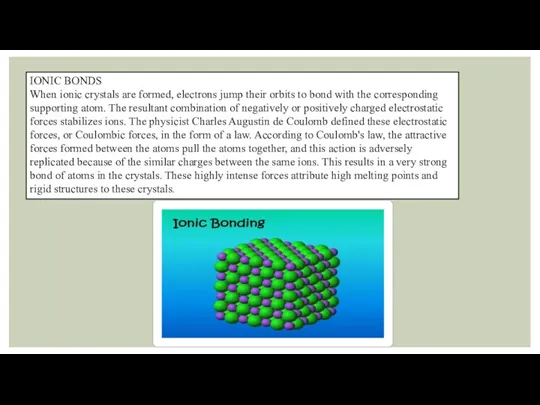
IONIC BONDS
When ionic crystals are formed, electrons jump their orbits to
IONIC BONDS
When ionic crystals are formed, electrons jump their orbits to
bond with the corresponding supporting atom. The resultant combination of negatively or positively charged electrostatic forces stabilizes ions. The physicist Charles Augustin de Coulomb defined these electrostatic forces, or Coulombic forces, in the form of a law. According to Coulomb's law, the attractive forces formed between the atoms pull the atoms together, and this action is adversely replicated because of the similar charges between the same ions. This results in a very strong bond of atoms in the crystals. These highly intense forces attribute high melting points and rigid structures to these crystals.
Слайд 5
Слайд 6
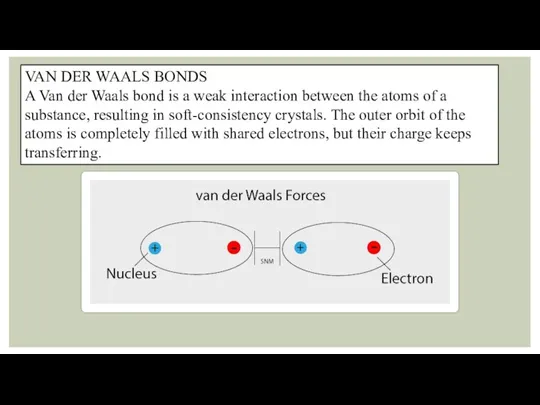
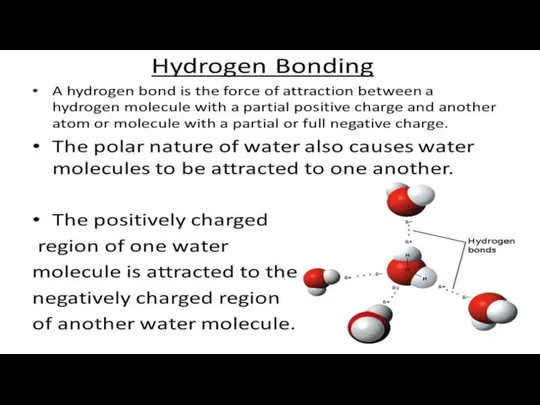
VAN DER WAALS BONDS
A Van der Waals bond is a weak
VAN DER WAALS BONDS
A Van der Waals bond is a weak
interaction between the atoms of a substance, resulting in soft-consistency crystals. The outer orbit of the atoms is completely filled with shared electrons, but their charge keeps transferring.
Слайд 7
Слайд 8


 Хімічний зв’язок. Ковалентний, йонний, металічний, водневий зв’язки, механізми їх утворення
Хімічний зв’язок. Ковалентний, йонний, металічний, водневий зв’язки, механізми їх утворення Анализ качества неорганических лекарственных средств количественно определяемых методами редоксиметрии
Анализ качества неорганических лекарственных средств количественно определяемых методами редоксиметрии Физические свойства минералов
Физические свойства минералов Химия элементов VIA группы
Химия элементов VIA группы Физические и химические явления
Физические и химические явления Методы контроля и анализа веществ
Методы контроля и анализа веществ Кремний, его физические и химические свойства
Кремний, его физические и химические свойства Синтез и химические модификации индиго
Синтез и химические модификации индиго Инертные газы
Инертные газы Гетероциклдік қосылыстар
Гетероциклдік қосылыстар Окислительно-восстановительные реакции. Основные закономерности окисления различных классов органических веществ
Окислительно-восстановительные реакции. Основные закономерности окисления различных классов органических веществ Признаки химических реакций
Признаки химических реакций Товары из пластмасс. Система маркировки пластика
Товары из пластмасс. Система маркировки пластика Повторение. Ионные уравнения реакции
Повторение. Ионные уравнения реакции Вcтуп до курсу Класифікації отрут і отруєнь. Токсикометрія
Вcтуп до курсу Класифікації отрут і отруєнь. Токсикометрія Адсорбция. Разделение однородных и неоднородных смесей
Адсорбция. Разделение однородных и неоднородных смесей Техника безопасности на уроках химии
Техника безопасности на уроках химии Кислоты. Состав кислот
Кислоты. Состав кислот Поликонденсация. Фенолформальдегидные смолы
Поликонденсация. Фенолформальдегидные смолы Методы пробоотбора воздуха. Лекция 2
Методы пробоотбора воздуха. Лекция 2 Химическая промышленность
Химическая промышленность Морские льды. Их классификация и закономерности движения
Морские льды. Их классификация и закономерности движения Начала органической химии
Начала органической химии Электроотрицательность химических элементов
Электроотрицательность химических элементов Молекулярные и немолекулярные вещества
Молекулярные и немолекулярные вещества Классификация химических реакций в органической и неорганической химии
Классификация химических реакций в органической и неорганической химии Алюминий
Алюминий Органическая химия
Органическая химия