Содержание
- 2. INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at
- 3. CONTENTS Introduction Electronic configuration Bonding & structure Atomic radius 1st Ionisation Energy Electrical conductivity Electronegativity Melting
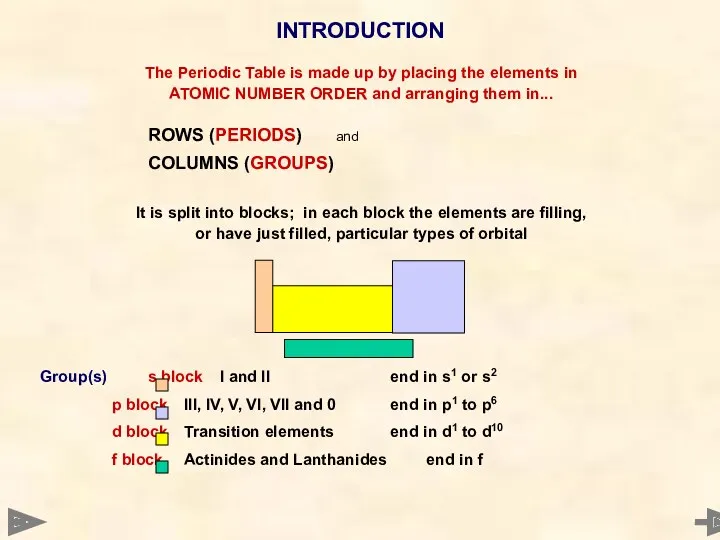
- 4. The Periodic Table is made up by placing the elements in ATOMIC NUMBER ORDER and arranging
- 5. The Periodic Table is made up by placing the elements in ATOMIC NUMBER ORDER and arranging
- 6. The Periodic Table is made up by placing the elements in ATOMIC NUMBER ORDER and arranging
- 7. The outer electron configuration is a periodic function... it repeats every so often Because many physical
- 8. The outer electron configuration is a periodic function... it repeats every so often Because many physical
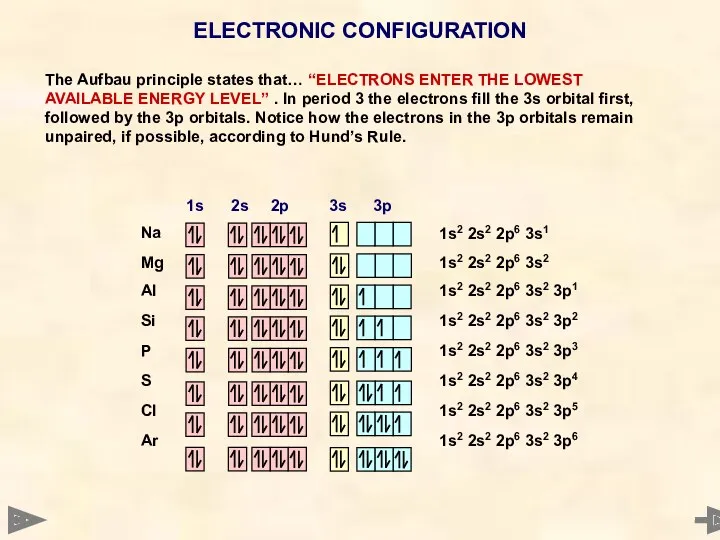
- 9. ELECTRONIC CONFIGURATION
- 10. ELECTRONIC CONFIGURATION The Aufbau principle states that… “ELECTRONS ENTER THE LOWEST AVAILABLE ENERGY LEVEL” . In
- 11. BONDING & STRUCTURE
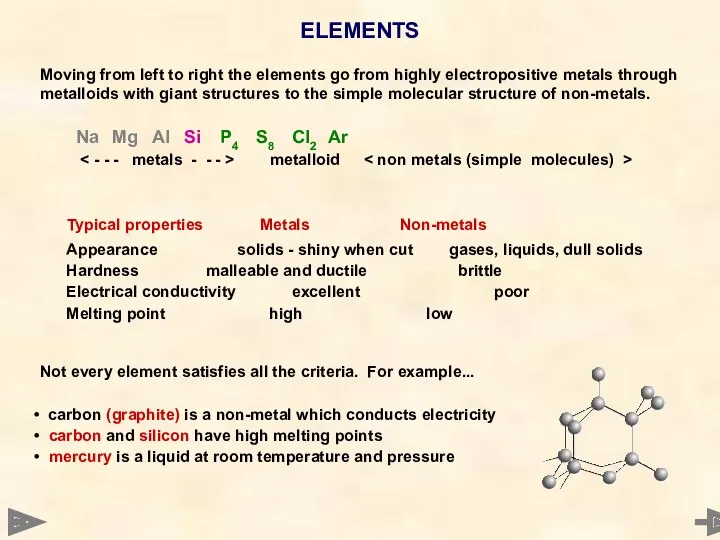
- 12. ELEMENTS Moving from left to right the elements go from highly electropositive metals through metalloids with
- 13. ELEMENTS Moving from left to right the elements go from highly electropositive metals through metalloids with
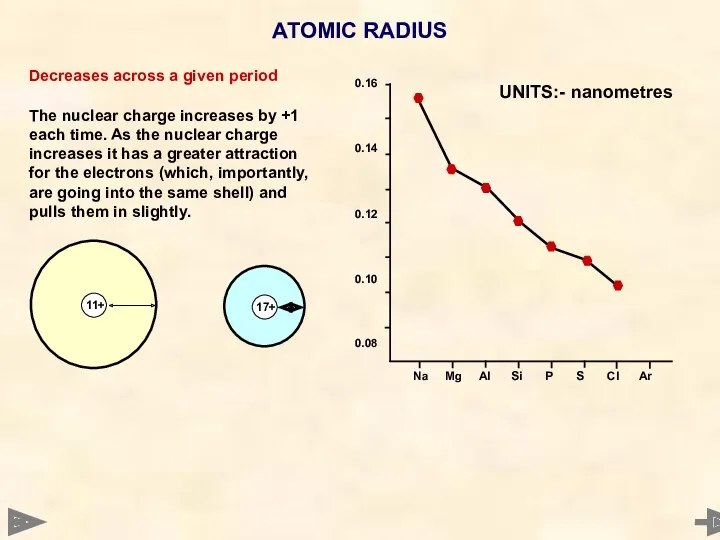
- 14. ATOMIC RADIUS
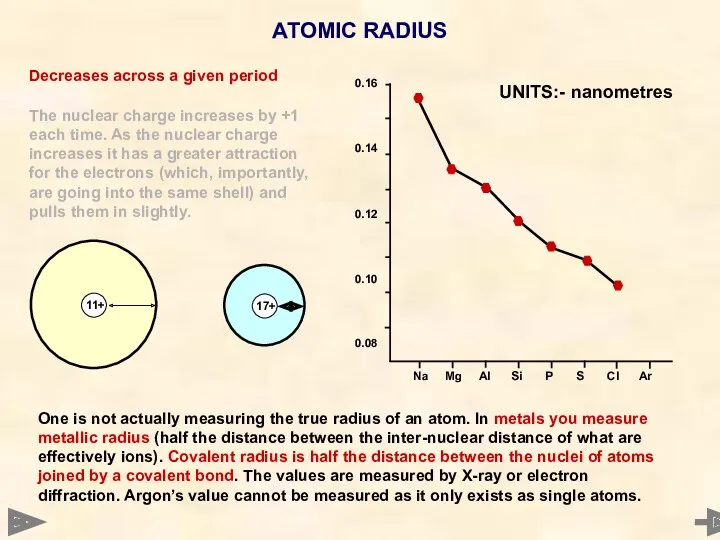
- 15. ATOMIC RADIUS Decreases across a given period The nuclear charge increases by +1 each time. As
- 16. ATOMIC RADIUS Decreases across a given period The nuclear charge increases by +1 each time. As
- 17. 1st IONISATION ENERGY
- 18. FIRST IONISATION ENERGY It is a measure of the energy required to remove an outer shell
- 19. FIRST IONISATION ENERGY It is a measure of the energy required to remove an outer shell
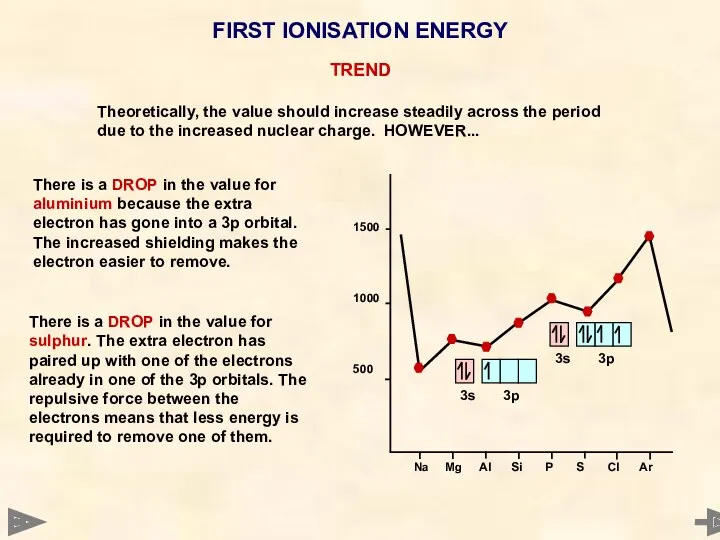
- 20. FIRST IONISATION ENERGY INCREASES across a period Nuclear charge increases by one each time. Each extra
- 21. FIRST IONISATION ENERGY There is a DROP in the value for sulphur. The extra electron has
- 22. ELECTRICAL CONDUCTIVITY
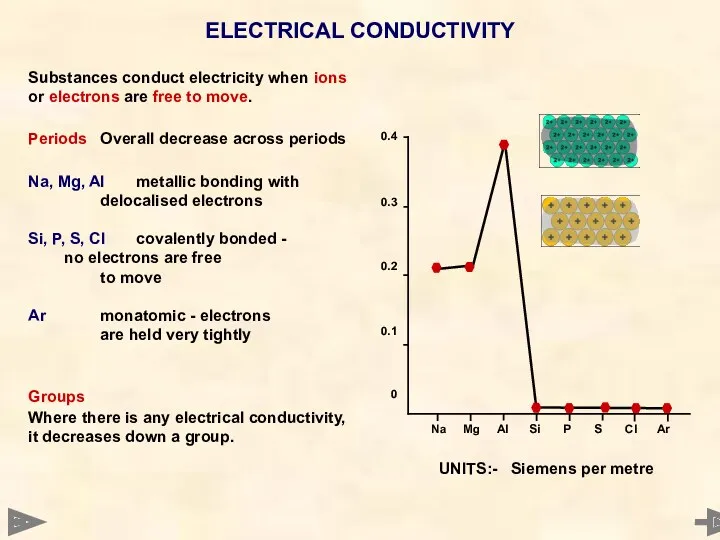
- 23. ELECTRICAL CONDUCTIVITY Substances conduct electricity when ions or electrons are free to move. Periods Overall decrease
- 24. ELECTRONEGATIVITY
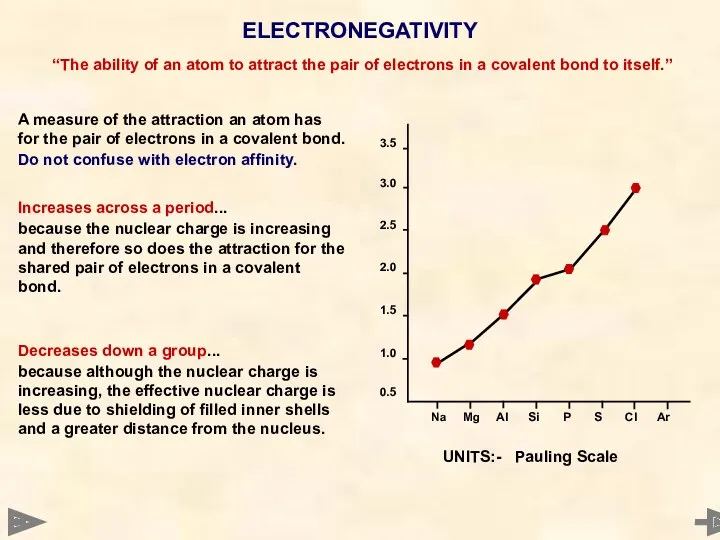
- 25. ELECTRONEGATIVITY A measure of the attraction an atom has for the pair of electrons in a
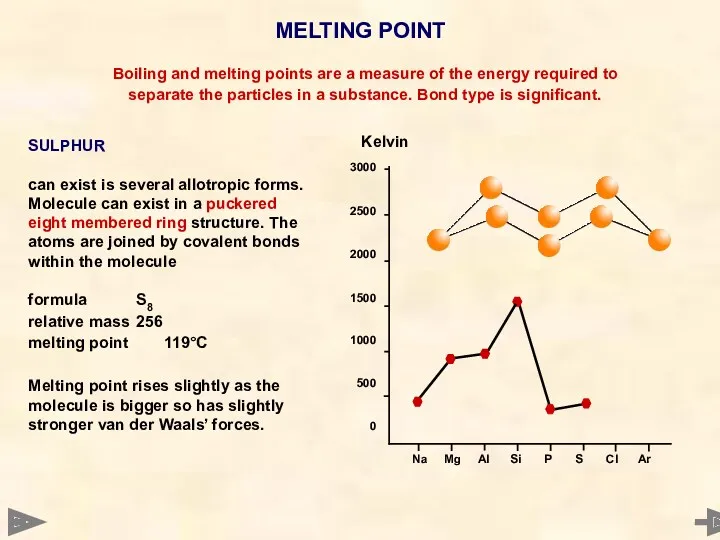
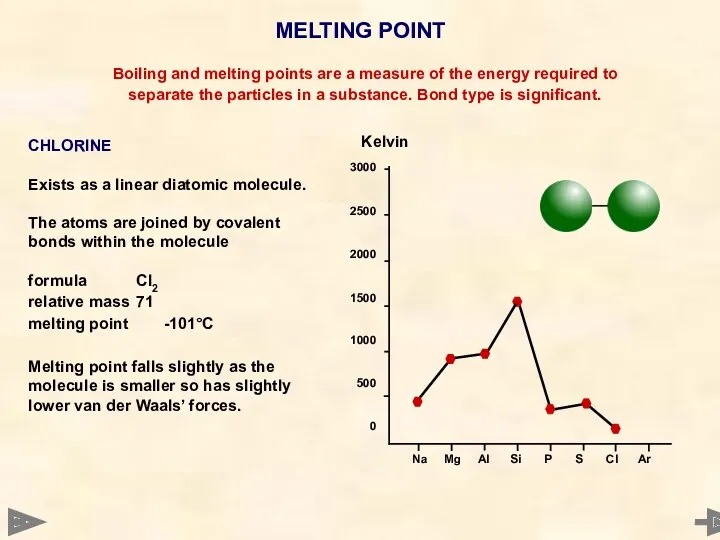
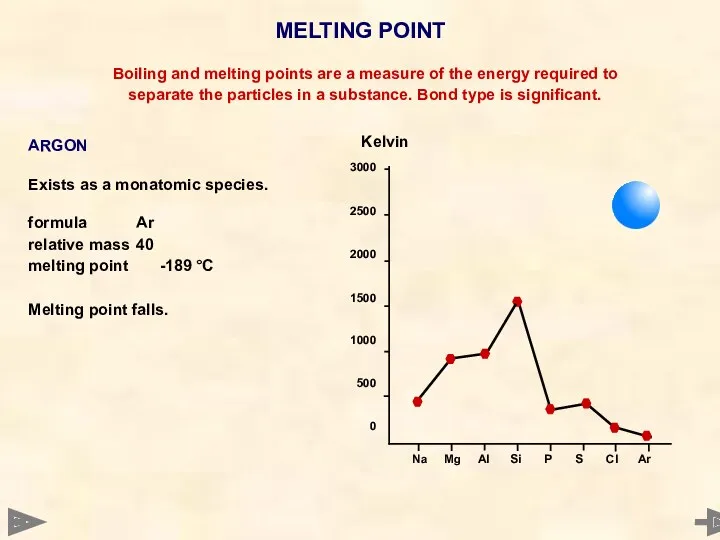
- 26. MELTING POINT
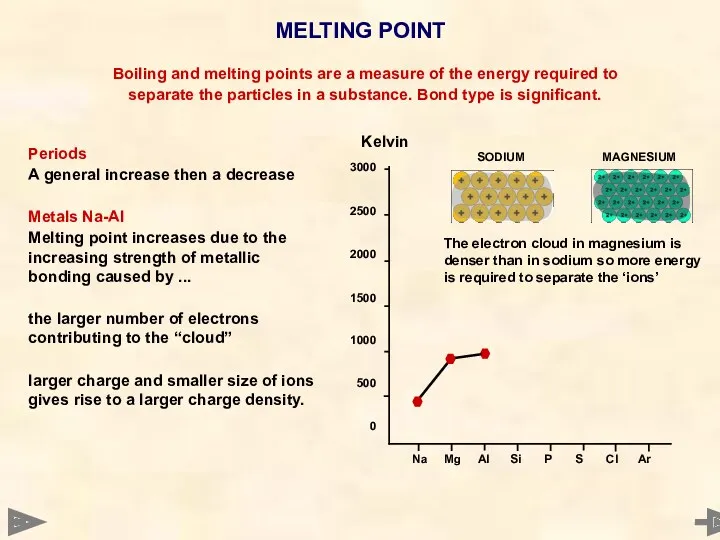
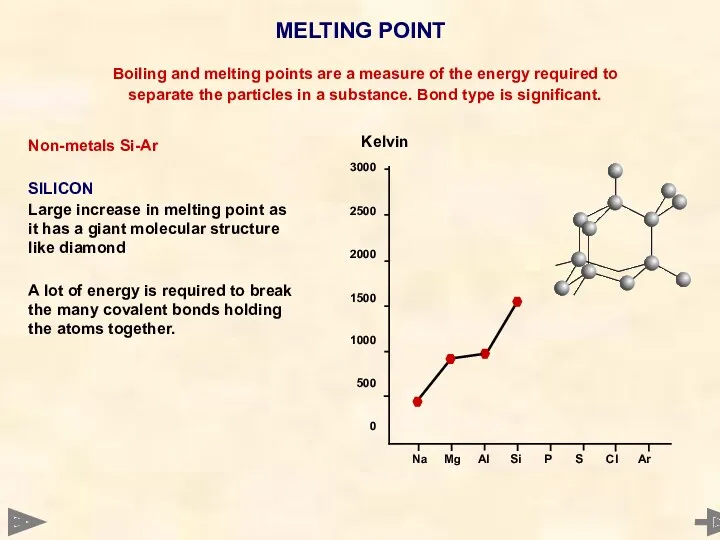
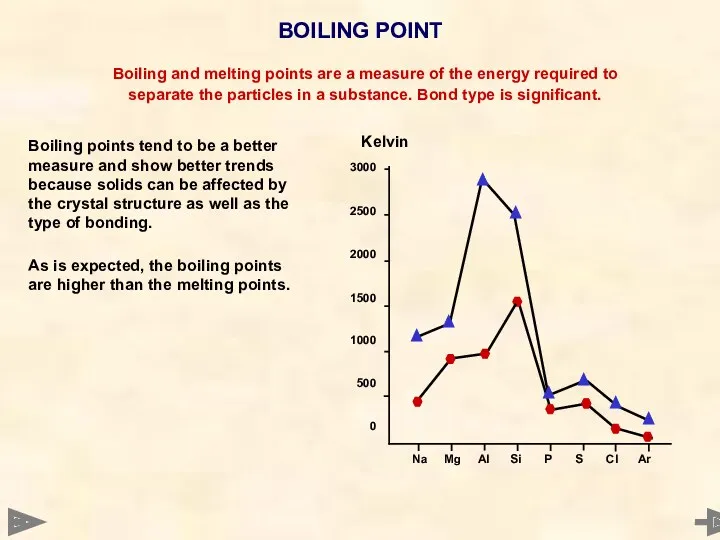
- 27. MELTING POINT 3000 2500 2000 1500 1000 500 0 Boiling and melting points are a measure
- 28. MELTING POINT 3000 2500 2000 1500 1000 500 0 Boiling and melting points are a measure
- 29. MELTING POINT 3000 2500 2000 1500 1000 500 0 Boiling and melting points are a measure
- 30. MELTING POINT 3000 2500 2000 1500 1000 500 0 Boiling and melting points are a measure
- 31. MELTING POINT 3000 2500 2000 1500 1000 500 0 Boiling and melting points are a measure
- 32. MELTING POINT 3000 2500 2000 1500 1000 500 0 Boiling and melting points are a measure
- 33. MELTING POINT Boiling and melting points are a measure of the energy required to separate the
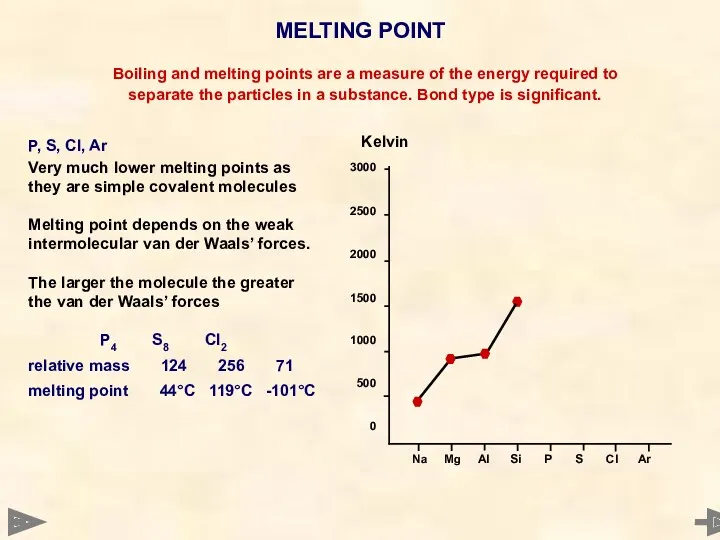
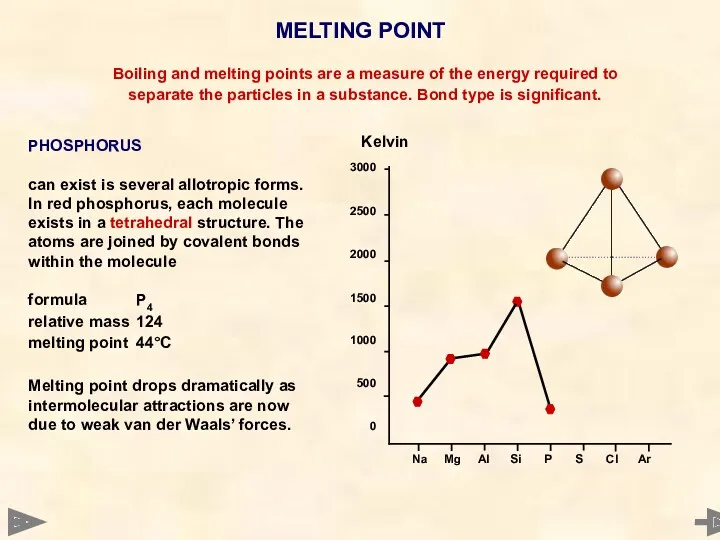
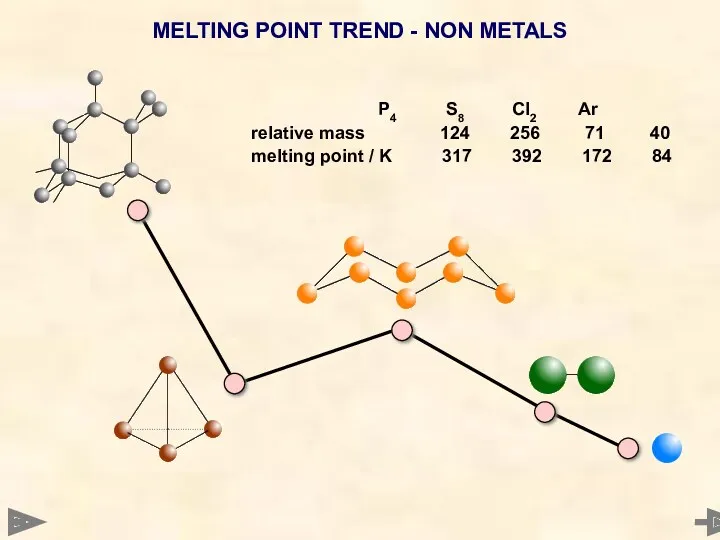
- 34. MELTING POINT TREND - NON METALS P4 S8 Cl2 Ar relative mass 124 256 71 40
- 35. 3000 2500 2000 1500 1000 500 0 Boiling points tend to be a better measure and
- 36. REVISION CHECK What should you be able to do? Recall and explain the trend in electronic
- 37. You need to go over the relevant topic(s) again Click on the button to return to
- 38. WELL DONE! Try some past paper questions
- 40. Скачать презентацию




















 VoIP – это просто
VoIP – это просто Кодирование звука
Кодирование звука Встроенный SQL. Два способа применения SQL в прикладных программах. (Лекция 8)
Встроенный SQL. Два способа применения SQL в прикладных программах. (Лекция 8) Гостехкомиссия и её роль в обеспечении информационной безопасности в РФ
Гостехкомиссия и её роль в обеспечении информационной безопасности в РФ Осуществление основных функций по администрированию баз данных
Осуществление основных функций по администрированию баз данных Пакеты прикладных программ
Пакеты прикладных программ Основные элементы языка программирования
Основные элементы языка программирования Одномерные массивы. Вставка и удаление элемента
Одномерные массивы. Вставка и удаление элемента Jizzax viloyat xalq ta’limi xodimlarini qayta tayyorlash va ularning malakasini oshirish hududiy markazi tomonidan tayyorlangan
Jizzax viloyat xalq ta’limi xodimlarini qayta tayyorlash va ularning malakasini oshirish hududiy markazi tomonidan tayyorlangan Библиотека PyGame. Создание 2D игры на языке программирования Python
Библиотека PyGame. Создание 2D игры на языке программирования Python Технология обработки текстовой информации. Текстовый редактор
Технология обработки текстовой информации. Текстовый редактор Составление алгоритмов
Составление алгоритмов Повторение прошедших тем. Логические выражения
Повторение прошедших тем. Логические выражения Разработка инструментального приложения для развертывания корпоративной распределенной информационной системы
Разработка инструментального приложения для развертывания корпоративной распределенной информационной системы Алгебра логики
Алгебра логики Программное обеспечение компьютера. Операционная система.
Программное обеспечение компьютера. Операционная система. Systemy informacyjne w zarządzaniu
Systemy informacyjne w zarządzaniu Безопасность в сети Интернет. Правила безопасности
Безопасность в сети Интернет. Правила безопасности Создание документов и основы редактирования в Microsoft WORD
Создание документов и основы редактирования в Microsoft WORD Беспроводные решения
Беспроводные решения Лабораторный практикум
Лабораторный практикум Приложения Java
Приложения Java Жизненный цикл программного обеспечения ИС
Жизненный цикл программного обеспечения ИС Двумерный массив
Двумерный массив Основные устройства системного блока
Основные устройства системного блока 40. Информационные ресурсы
40. Информационные ресурсы Блоктік шифр үшін кеңейтілген дифференциалдық криптоталдау жасау
Блоктік шифр үшін кеңейтілген дифференциалдық криптоталдау жасау Условный оператор
Условный оператор