Содержание
- 2. HEMOPOIESIS. The formation of blood cells in the penatal life is named Hemopoiesis (Gr. haima, blood
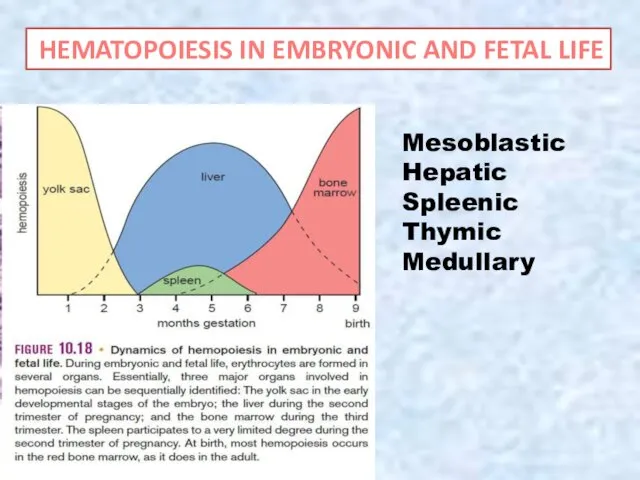
- 3. Mesoblastic Hepatic Spleenic Thymic Medullary HEMATOPOIESIS IN EMBRYONIC AND FETAL LIFE
- 4. Theories of hematopoiesis The monophyletic theory suggests that a pluripotent stem cell (CFU-S) can form all
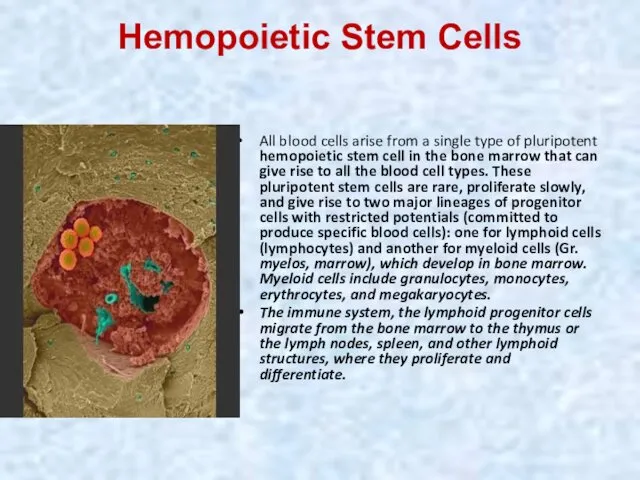
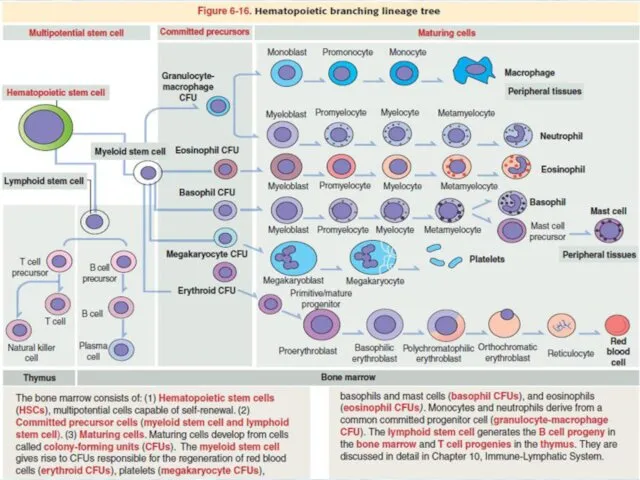
- 5. Hemopoietic Stem Cells All blood cells arise from a single type of pluripotent hemopoietic stem cell
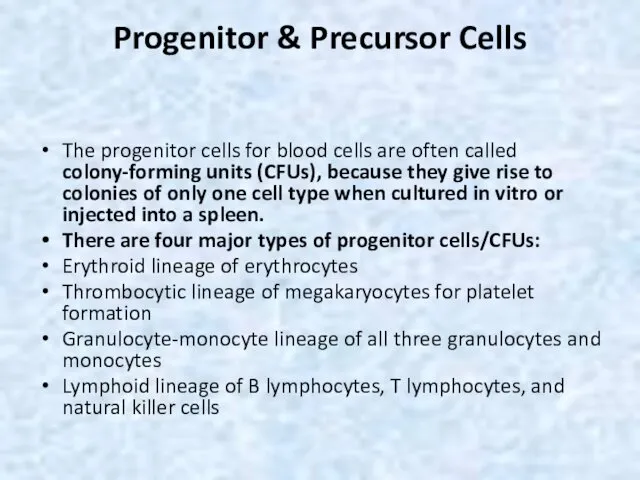
- 6. Progenitor & Precursor Cells The progenitor cells for blood cells are often called colony-forming units (CFUs),
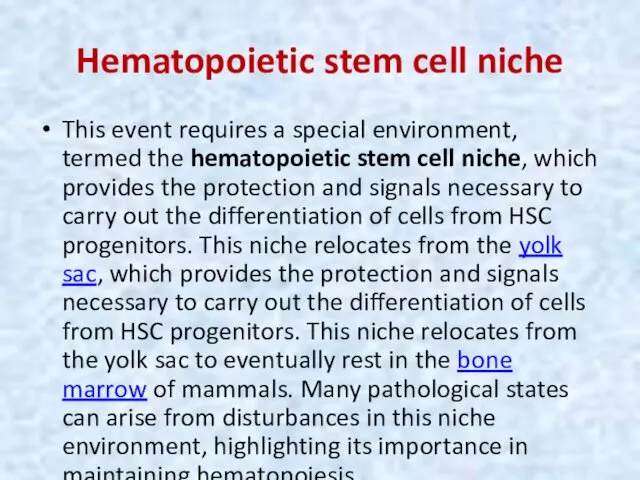
- 8. Hematopoietic stem cell niche This event requires a special environment, termed the hematopoietic stem cell niche,
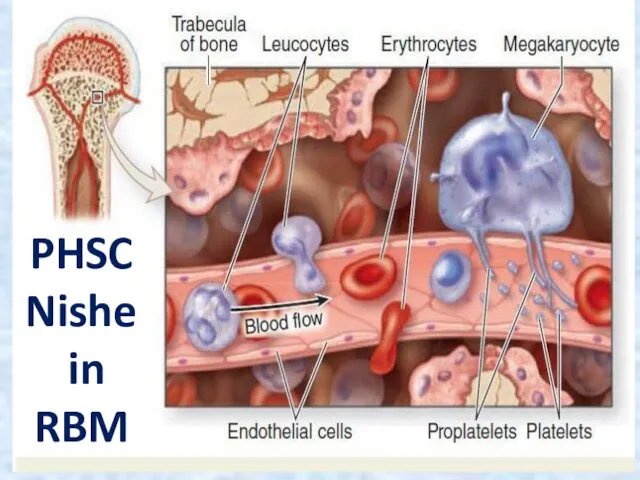
- 9. PHSC Nishe in RBM
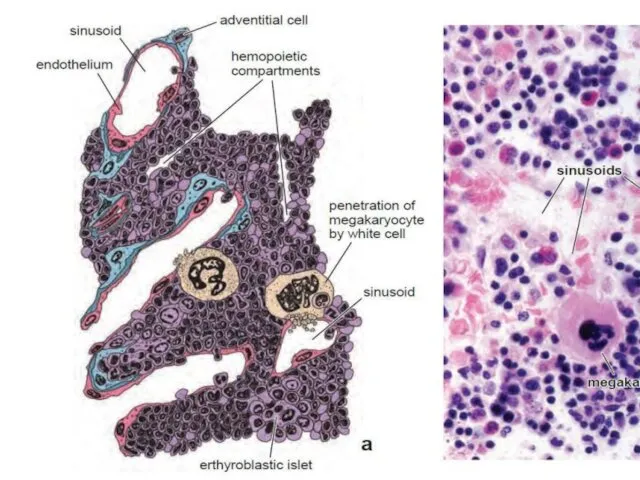
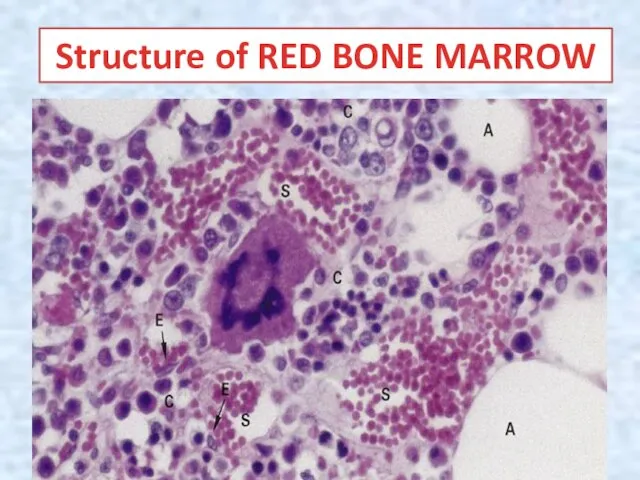
- 10. Red bone marrow Red bone marrow contains a reticular connective tissue stroma (Gr. stroma, bed), hemopoietic
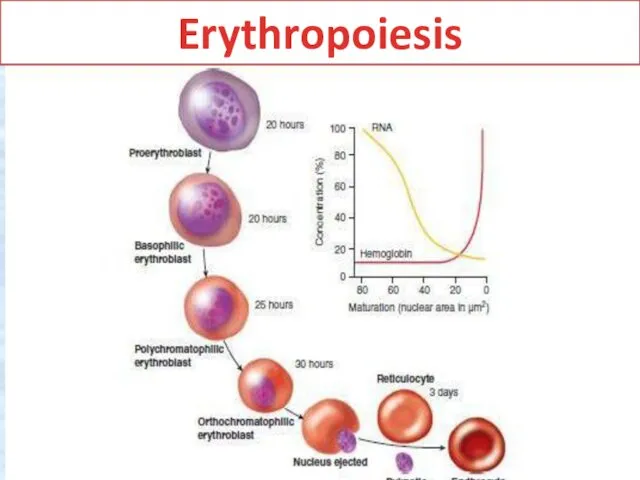
- 12. Erythropoiesis Erythropoiesis. In healthy adults, erythropoiesis (red blood cell formation) occurs exclusively in bone marrow. Erythrocytes
- 13. Erythropoiesis
- 14. Structure of RED BONE MARROW
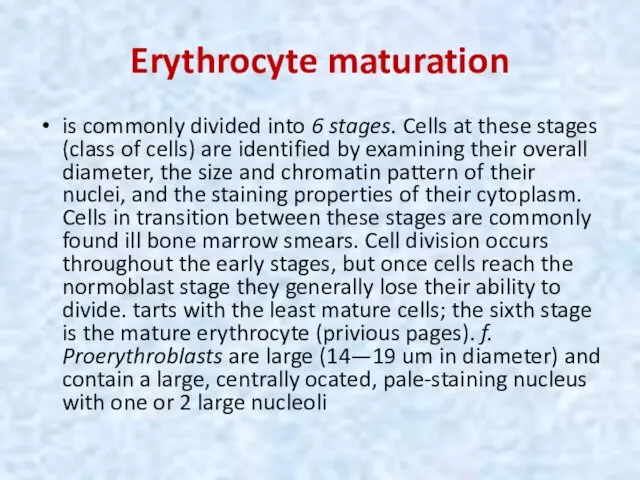
- 15. Erythrocyte maturation is commonly divided into 6 stages. Cells at these stages (class of cells) are
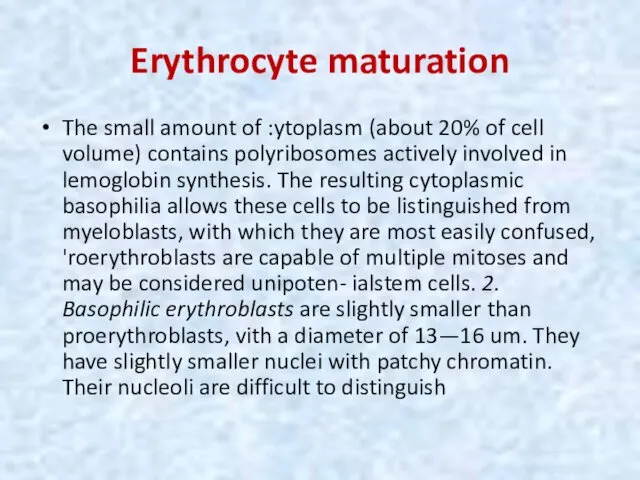
- 16. Erythrocyte maturation The small amount of :ytoplasm (about 20% of cell volume) contains polyribosomes actively involved
- 17. Erythrocyte maturation The cytoplasm is more intensely >asophilic, typically staining a deep royal blue. A prominent,
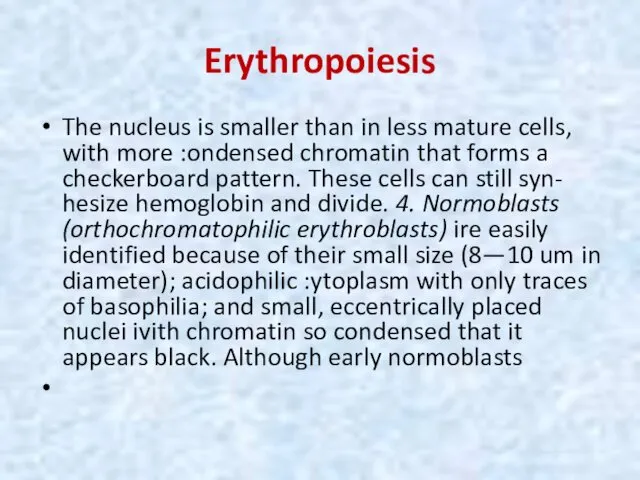
- 18. Erythropoiesis The nucleus is smaller than in less mature cells, with more :ondensed chromatin that forms
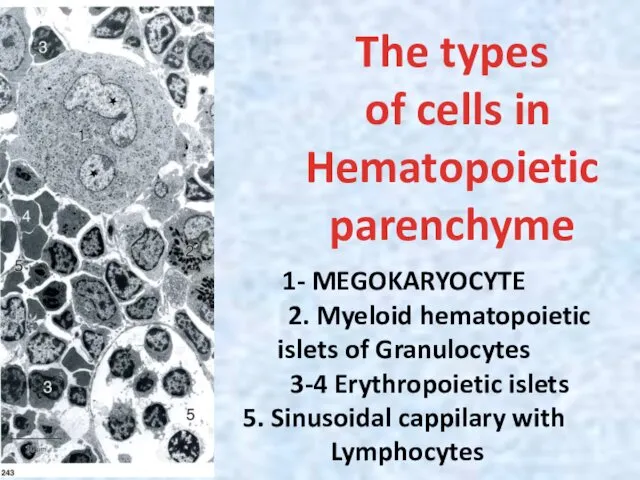
- 19. The types of cells in Hematopoietic parenchyme 1- MEGOKARYOCYTE 2. Myeloid hematopoietic islets of Granulocytes 3-4
- 20. Leukopoiesis Leukopoiesis (white blood cell formation) encompasses both granulopoiesis and agranulopoiesis. Leukopoietic CFUs that have been
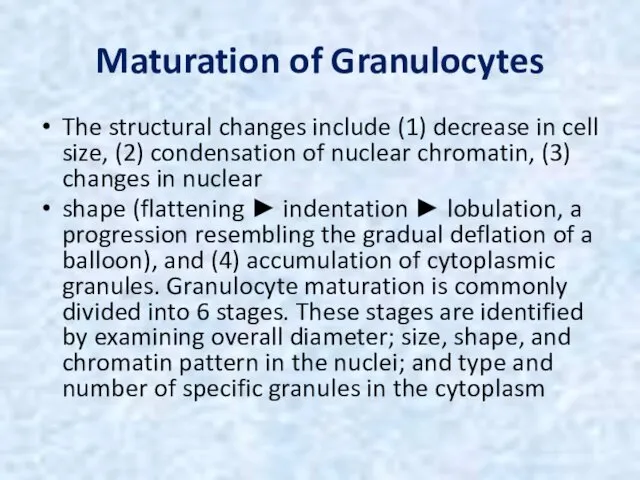
- 21. Maturation of Granulocytes The structural changes include (1) decrease in cell size, (2) condensation of nuclear
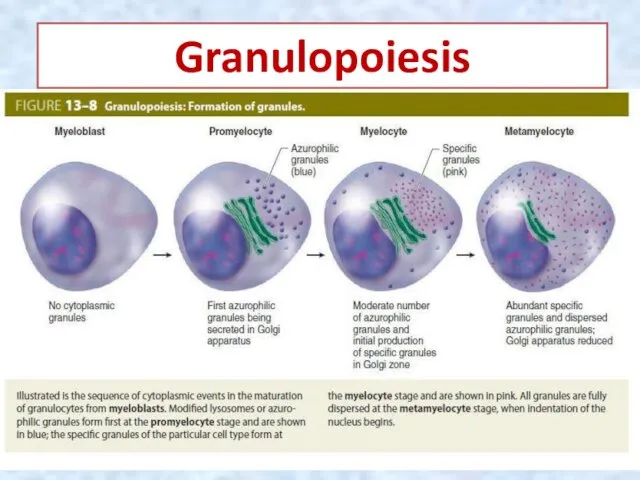
- 22. Granulopoiesis
- 23. Myeloblasts, the earliest recognizable granulocyte precursors, are about 15 um in diameter. Promyelocytes are larger than
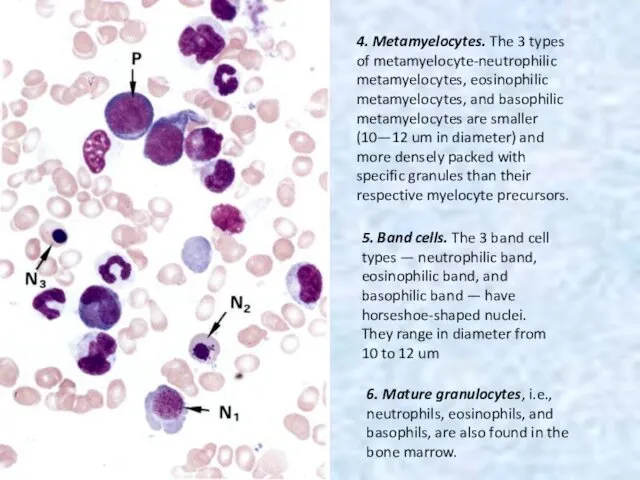
- 24. 4. Metamyelocytes. The 3 types of metamyelocyte-neutrophilic metamyelocytes, eosinophilic metamyelocytes, and basophilic metamyelocytes are smaller (10—12
- 25. Agranulopoiesis Agranulopoiesis: agranulocytes (monocytes and lymphocytes), like the other blood cell types, derive from CFU-Ss. The
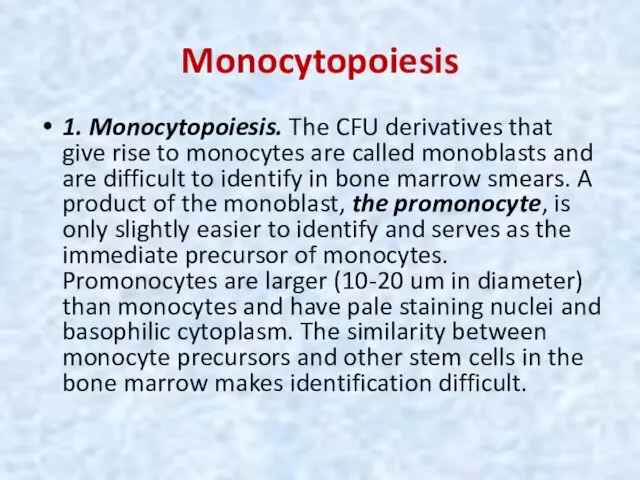
- 26. Monocytopoiesis 1. Monocytopoiesis. The CFU derivatives that give rise to monocytes are called monoblasts and are
- 27. Lymphopoiesis 2. Lymphopoiesis. In adults, lymphopoiesis occurs mainly in lymphoid tissues and organs and to a
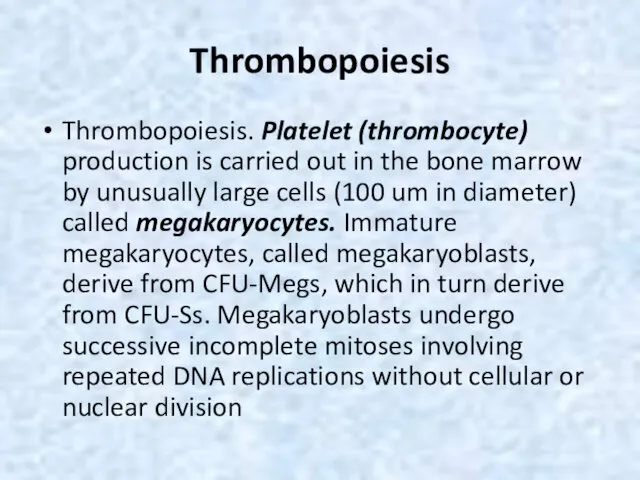
- 28. Thrombopoiesis Thrombopoiesis. Platelet (thrombocyte) production is carried out in the bone marrow by unusually large cells
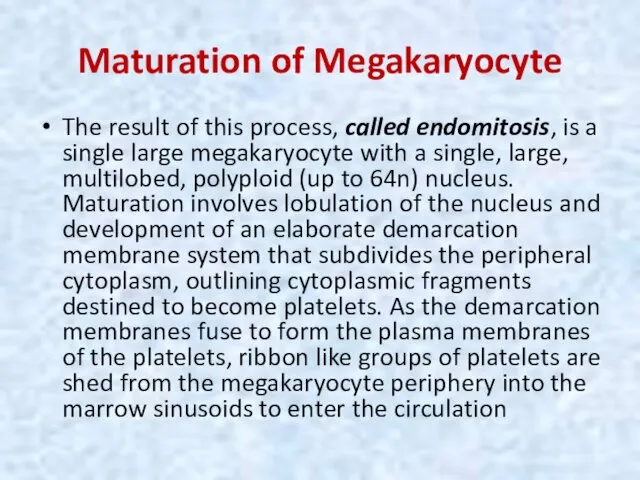
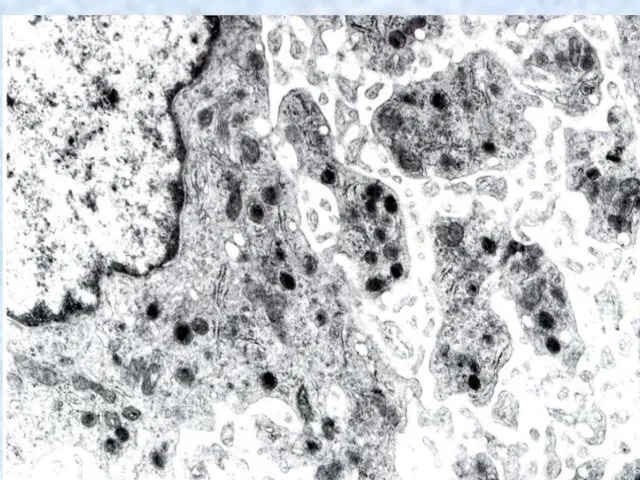
- 29. Maturation of Megakaryocyte The result of this process, called endomitosis, is a single large megakaryocyte with
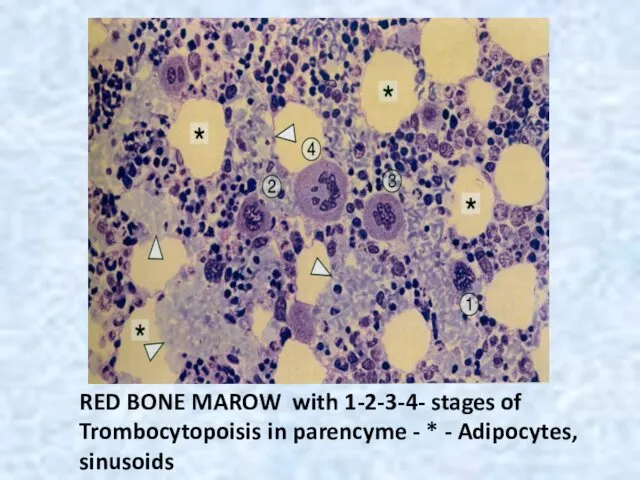
- 31. RED BONE MAROW with 1-2-3-4- stages of Trombocytopoisis in parencyme - * - Adipocytes, sinusoids
- 32. Regulation of hematopoiesis involves specific colony-stimulating factors (CSFs) such as erythropoietin, leukopoietin and thrombopoietin. These hormones
- 33. Erythropoietin/ Thrombopoietin CSFs are also responsible for the stimulation of cell division and for the differentiation
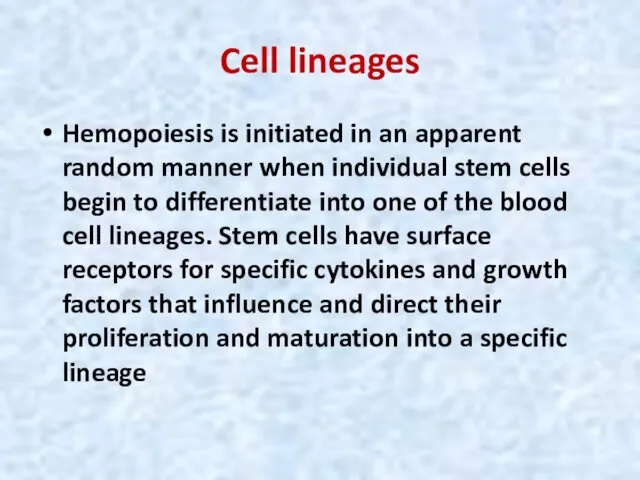
- 34. Cell lineages Hemopoiesis is initiated in an apparent random manner when individual stem cells begin to
- 35. INTERACTION OF IMMUNE CELLS The immune system of an organism consist of two basic ingredients: organs
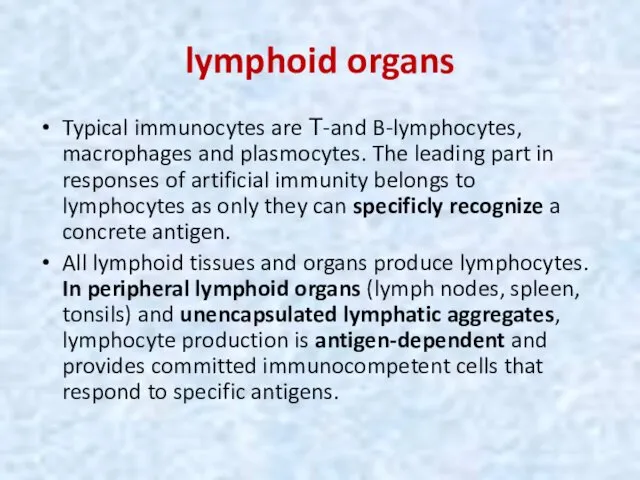
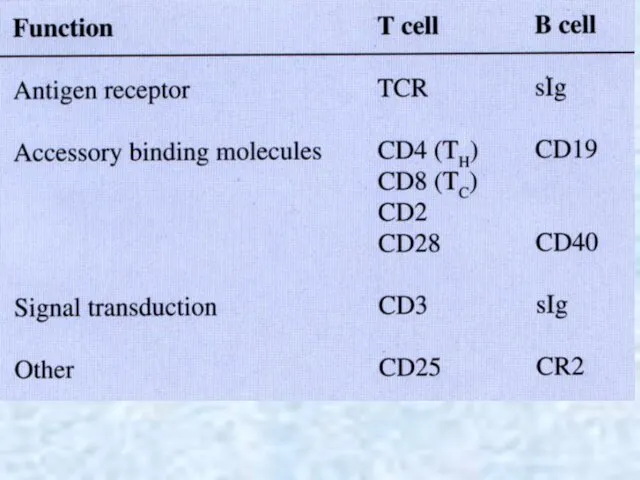
- 36. lymphoid organs Typical immunocytes are Т-and B-lymphocytes, macrophages and plasmocytes. The leading part in responses of
- 37. Central lymphoid organs In central lymphoid organs (thymus, bone marrow, bursa of Fabricius [in birds]), lymphocyte
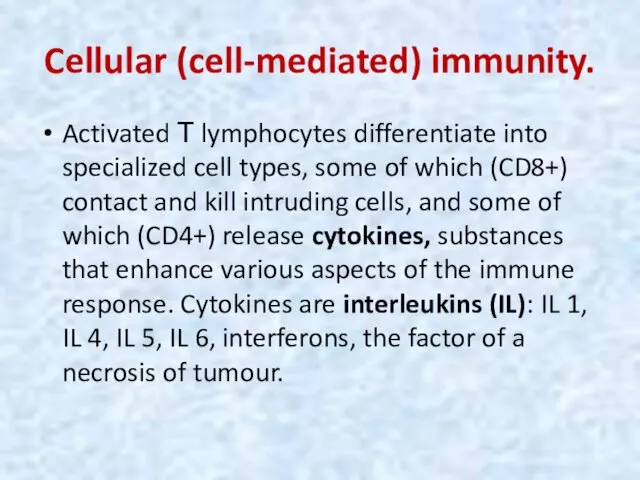
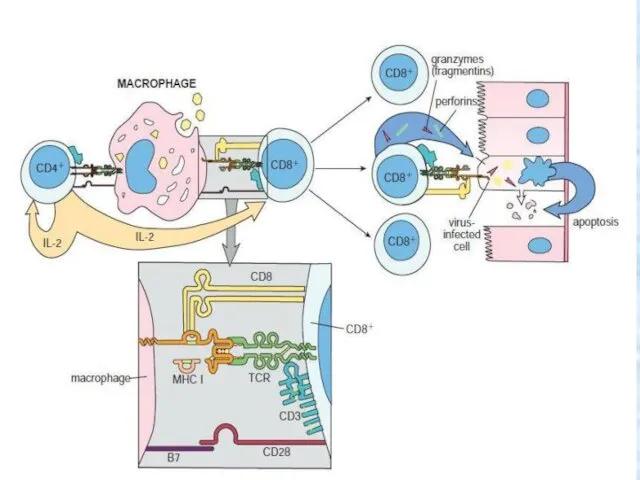
- 38. Cellular (cell-mediated) immunity. Activated Т lymphocytes differentiate into specialized cell types, some of which (CD8+) contact
- 39. Humoral immunity Activated В lymphocytes differentiate into plasma cells that secrete antigen-binding immunoglobulins (antibodies), which circulate
- 40. Specificity Specificity. An ability to respond to one type of infection (chicken pox) does not imply
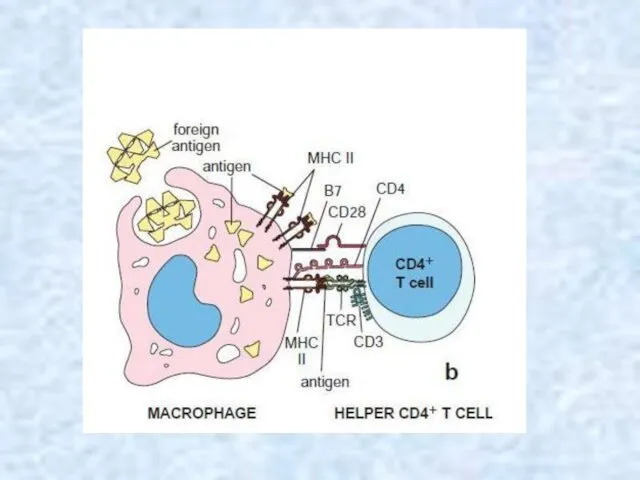
- 41. Lymphocyte programming and activation This multistep process is outlined below. 1. Cells of mesodermal origin are
- 43. Selectively stimulation 3. Not all lymphocytes can respond to all antigens. Our ability to respond to
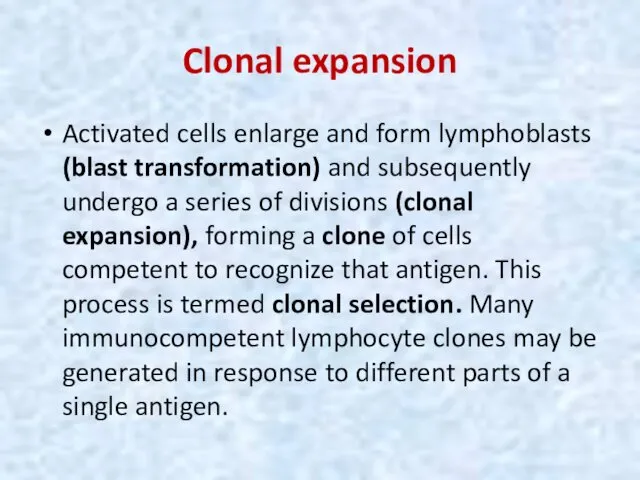
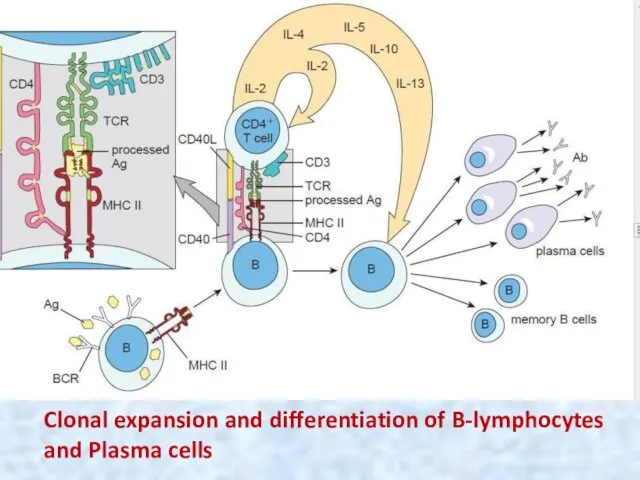
- 44. Clonal expansion Activated cells enlarge and form lymphoblasts (blast transformation) and subsequently undergo a series of
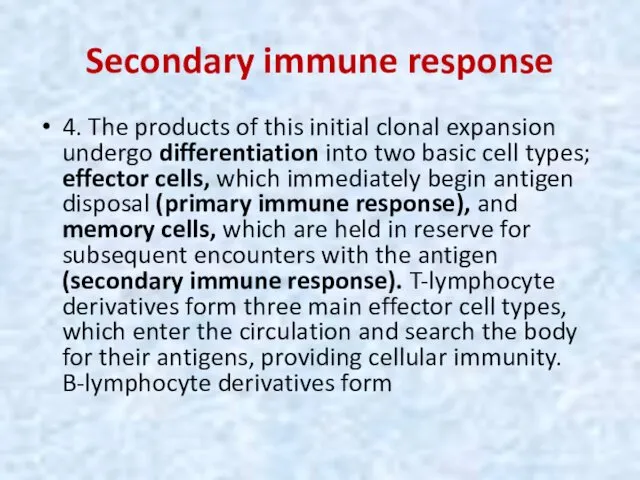
- 46. Secondary immune response 4. The products of this initial clonal expansion undergo differentiation into two basic
- 47. Clonal expansion and differentiation of B-lymphocytes and Plasma cells
- 48. 5. When the same antigen is again encountered, memory cells generated during the initial clonal selection
- 49. Antigens These are foreign (nonself) substances that are able to elicit an immune response (cellular, humoral,
- 50. The specific part of an antigen that elicits the immune response (and to which the antibodies
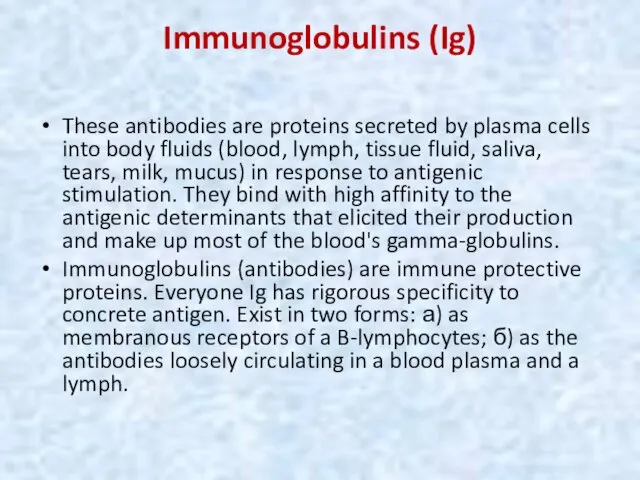
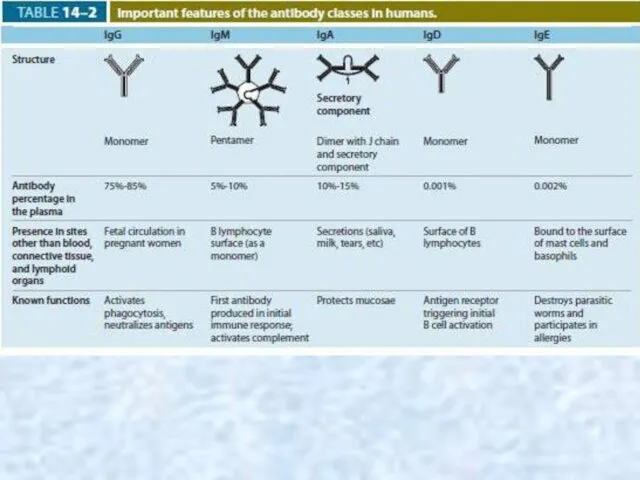
- 51. Immunoglobulins (Ig) These antibodies are proteins secreted by plasma cells into body fluids (blood, lymph, tissue
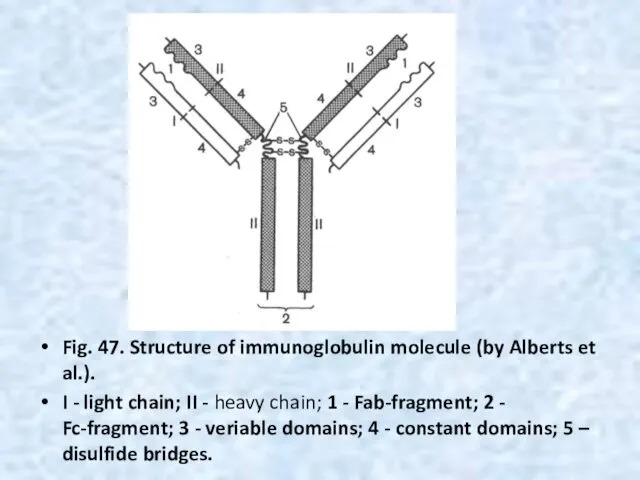
- 52. Fig. 47. Structure of immunoglobulin molecule (by Alberts et al.). I - light chain; II -
- 54. The mechanism of cytolytic activity of the Т-killer (Т-cytotoxic lymphocyte) on a cell - target. T
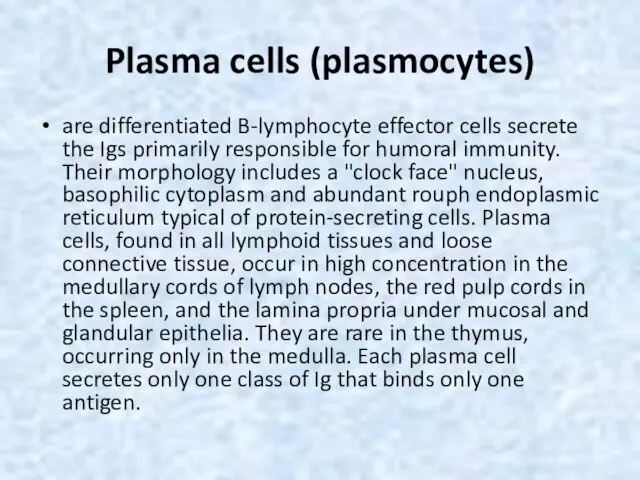
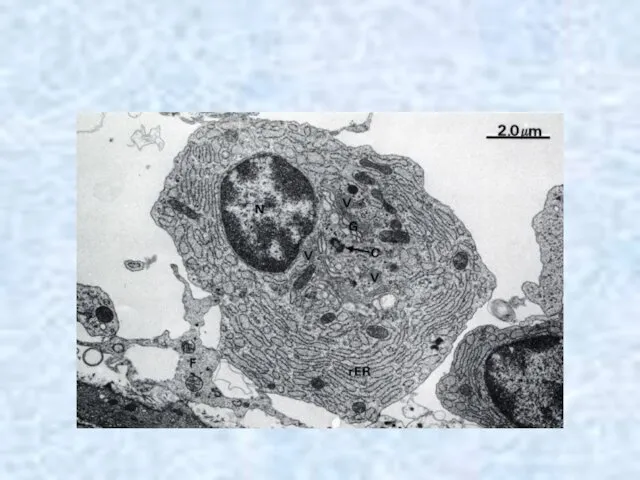
- 55. Plasma cells (plasmocytes) are differentiated B-lymphocyte effector cells secrete the Igs primarily responsible for humoral immunity.
- 58. THANK YOU
- 60. Скачать презентацию






















 Вирусты. Гепатит В. Эпидемиологиясы
Вирусты. Гепатит В. Эпидемиологиясы Реабилитация при Инфаркте Миокарда
Реабилитация при Инфаркте Миокарда Отбеливание зубов
Отбеливание зубов Базовая модель медико-социальной работы
Базовая модель медико-социальной работы Дизайны клинических исследований
Дизайны клинических исследований Составление схемы диспансерного наблюдений курируемых хронических больных
Составление схемы диспансерного наблюдений курируемых хронических больных Общая анестезия. . Виды общей анестезии. Клиническая фармакология средств применяемых в анестезиологическом пособии
Общая анестезия. . Виды общей анестезии. Клиническая фармакология средств применяемых в анестезиологическом пособии Нарушения речи при детских неврозах. Мутизм, сурдомутизм, заикание
Нарушения речи при детских неврозах. Мутизм, сурдомутизм, заикание Профессиональные нейротоксикозы
Профессиональные нейротоксикозы Жұқпалы үрдіс патофизиологиясы. Сепсис және сепсистік сілейменің патогенезі
Жұқпалы үрдіс патофизиологиясы. Сепсис және сепсистік сілейменің патогенезі Лечебное питание при различных нарушениях обмена веществ
Лечебное питание при различных нарушениях обмена веществ Электрическая ось сердца и электрическая позиция сердца
Электрическая ось сердца и электрическая позиция сердца Biological Therapy in Psychiatry
Biological Therapy in Psychiatry Облитерирующий атеросклероз брюшной аорты и артерий нижних конечностей
Облитерирующий атеросклероз брюшной аорты и артерий нижних конечностей Жүрек гликозидтері
Жүрек гликозидтері Визуальная диагностика при гипоталамо-гипофизарном ожирении
Визуальная диагностика при гипоталамо-гипофизарном ожирении Топографическая анатомия нижней конечности. Области мышечной и сосудистой лакуны, бедра, подколенная ямка, ягодичная область
Топографическая анатомия нижней конечности. Области мышечной и сосудистой лакуны, бедра, подколенная ямка, ягодичная область Внелегочной туберкулез
Внелегочной туберкулез Ұрықтың туа пайда болған орталық жүйке жүйесінің ақаулары
Ұрықтың туа пайда болған орталық жүйке жүйесінің ақаулары Ультразвуковая терапия
Ультразвуковая терапия Морфология и цикл развития дизентерийной амебы
Морфология и цикл развития дизентерийной амебы Дифференциальный диагноз анемий
Дифференциальный диагноз анемий Тәуліктік PH-метрия
Тәуліктік PH-метрия Заболевания простаты
Заболевания простаты Абдоминальный массаж
Абдоминальный массаж Электронное здравоохранение. Медицинские информационные системы
Электронное здравоохранение. Медицинские информационные системы Организация работы с непригодными для медицинского использования лекарственными средствами
Организация работы с непригодными для медицинского использования лекарственными средствами Алгоритм диагностики заболеваний органов дыхания
Алгоритм диагностики заболеваний органов дыхания