- Главная
- Без категории
- Biochemical and genetic markers

Содержание
- 2. Introduction In all countries, women above a fixed cut-off age were regarded as at high enough
- 3. First Biochemical Marker In 1984, Merkatz et al. published the association of low maternal serum α-fetoprotein
- 4. What is it AFP? it was used to screen for neural tube defects, at 16–18 weeks'
- 5. A brief history of AFP Maternal serum AFP screening for aneuploidy was widely adopted and had
- 6. FIRST HIGHLY DISCRIMINATORY MARKER Human chorionic gonadotropin (hCG). This molecule is a heterodimer consisting of α
- 8. Power of uE3 There have been disputes over whether to include uE3 as a third parameter.
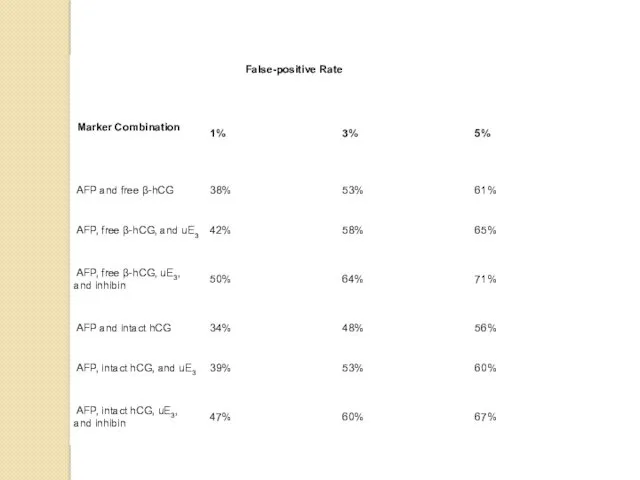
- 9. MULTIPLE BIOCHEMICAL MARKERS The discovery that hCG was a marker was quickly followed by another second
- 11. Another long promising but yet to be fulfilled marker was the search for fetal cells in
- 12. SEQUENTIAL SCREENING METHODS Three types of sequential policy have received attention. The first to be proposed
- 13. A second approach (step-wise test) begins with first trimester PAPP-A, free β-hCG or intact hCG, and
- 14. Sequential screening policies: predicted* detection rate for a given false-positive rate
- 16. Скачать презентацию
Introduction
In all countries, women above a fixed cut-off age were regarded
Introduction
In all countries, women above a fixed cut-off age were regarded
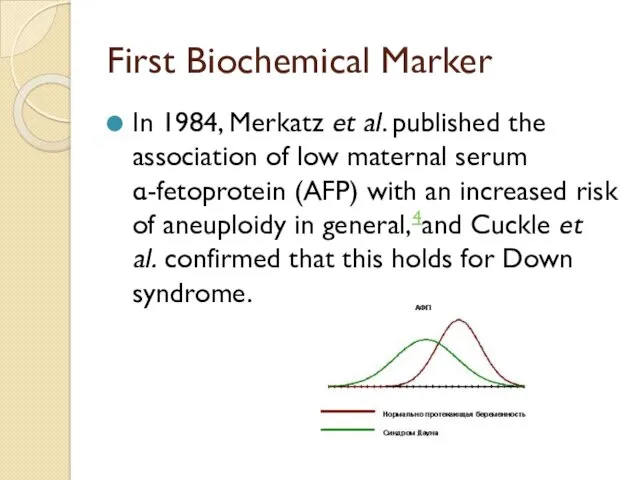
First Biochemical Marker
In 1984, Merkatz et al. published the association of low
First Biochemical Marker
In 1984, Merkatz et al. published the association of low
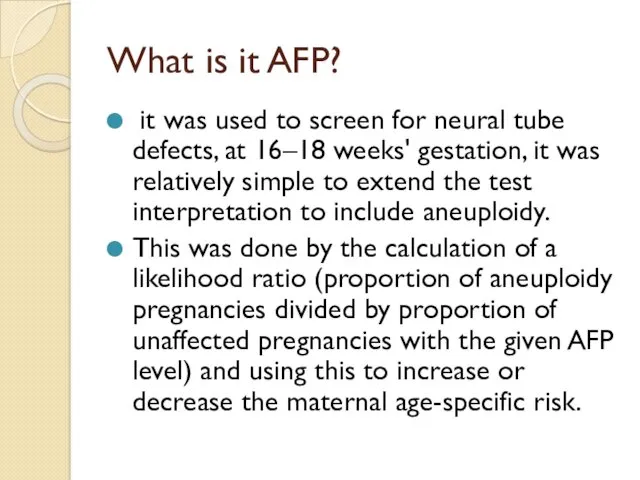
What is it AFP?
it was used to screen for neural
What is it AFP?
it was used to screen for neural
This was done by the calculation of a likelihood ratio (proportion of aneuploidy pregnancies divided by proportion of unaffected pregnancies with the given AFP level) and using this to increase or decrease the maternal age-specific risk.
A brief history of AFP
Maternal serum AFP screening for aneuploidy was
A brief history of AFP
Maternal serum AFP screening for aneuploidy was
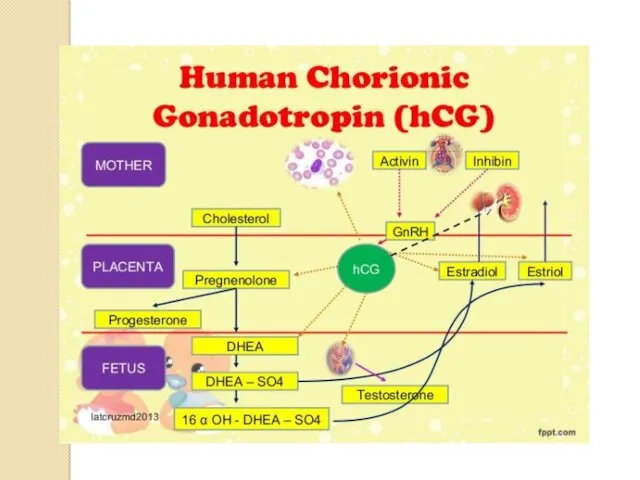
FIRST HIGHLY DISCRIMINATORY MARKER
Human chorionic gonadotropin (hCG).
This molecule is a heterodimer
FIRST HIGHLY DISCRIMINATORY MARKER
Human chorionic gonadotropin (hCG).
This molecule is a heterodimer
Power of uE3
There have been disputes over whether to include uE3 as
Power of uE3
There have been disputes over whether to include uE3 as
MULTIPLE BIOCHEMICAL MARKERS
The discovery that hCG was a marker was quickly
MULTIPLE BIOCHEMICAL MARKERS
The discovery that hCG was a marker was quickly
Another long promising but yet to be fulfilled marker was the
Another long promising but yet to be fulfilled marker was the
SEQUENTIAL SCREENING METHODS
Three types of sequential policy have received attention. The
SEQUENTIAL SCREENING METHODS
Three types of sequential policy have received attention. The
A second approach (step-wise test) begins with first trimester PAPP-A, free β-hCG
A second approach (step-wise test) begins with first trimester PAPP-A, free β-hCG
A third policy, more efficient than the other types, is called the contingent test. This begins with first trimester PAPP-A, free β-hCG or intact hCG, and NT. Women with very high risk are offered immediate invasive prenatal diagnosis and only those with borderline risks are offered second trimester AFP, uE3, free β-hCG or intact hCG, and inhibin; their risk is estimated from all seven markers. The borderline is chosen so that a large proportion of women have early assurance. This group has such a low risk that it is very unlikely that further markers will lead to a final high risk result.
Sequential screening policies: predicted* detection rate for a given false-positive rate
Sequential screening policies: predicted* detection rate for a given false-positive rate






 Автоматизации твердого звука [ Р] в словах со стечением согласных
Автоматизации твердого звука [ Р] в словах со стечением согласных Газовая сварка стали
Газовая сварка стали Визуальные словари для изучения английского языка
Визуальные словари для изучения английского языка Презентация по проекту.pptx
Презентация по проекту.pptx Исследовательская работа по краеведению Топонимы Белогорского района Амурской области
Исследовательская работа по краеведению Топонимы Белогорского района Амурской области Введение в зоологию. Подцарство Одноклеточные или Простейшие (Protozoa)
Введение в зоологию. Подцарство Одноклеточные или Простейшие (Protozoa) Игры для адаптации детей младшего возраста в период логопедического мониторинга.
Игры для адаптации детей младшего возраста в период логопедического мониторинга. Презентация к у року в 6 классе на тему Атмосферные осадки
Презентация к у року в 6 классе на тему Атмосферные осадки СССР в последние десятилетия советской власти (1965 - 1991)
СССР в последние десятилетия советской власти (1965 - 1991) 04 (2)
04 (2) Расчет короба под динамик DD3512H
Расчет короба под динамик DD3512H Дорожный фонд Республики Беларусь
Дорожный фонд Республики Беларусь Совершенствование коррекционно-развивающей среды логопедического кабинета в условиях реализации ФГОС
Совершенствование коррекционно-развивающей среды логопедического кабинета в условиях реализации ФГОС Oil industry Jargon
Oil industry Jargon Биохимия крови
Биохимия крови Технолігїї підвищення продуктивності процесорів
Технолігїї підвищення продуктивності процесорів Презентация Развитие творческих качеств педагога как условие развития личностных качеств и творческих способностей дошкольников
Презентация Развитие творческих качеств педагога как условие развития личностных качеств и творческих способностей дошкольников Питание в туристском походе
Питание в туристском походе Оценка состояния плода во время беременности и родов
Оценка состояния плода во время беременности и родов История Казахстана
История Казахстана Нормативно-правовые акты по охране труда
Нормативно-правовые акты по охране труда Прославлення і хвала
Прославлення і хвала лягушонок _ cvc words _ by Artem Morozov
лягушонок _ cvc words _ by Artem Morozov Викторина: Цветы
Викторина: Цветы Общение как социально-психологическая категория
Общение как социально-психологическая категория Экономическая политика Петра I
Экономическая политика Петра I Ю.А.Гагарин и отряд первых космонавтов на Саратовской земле
Ю.А.Гагарин и отряд первых космонавтов на Саратовской земле Педагогический совет.
Педагогический совет.