Содержание
- 2. HISTORY OF THE ATOM 460 BC Democritus develops the idea of atoms he pounded up materials
- 3. HISTORY OF THE ATOM 1808 John Dalton suggested that all matter was made up of tiny
- 4. HISTORY OF THE ATOM 1898 Joseph John Thompson found that atoms could sometimes eject a far
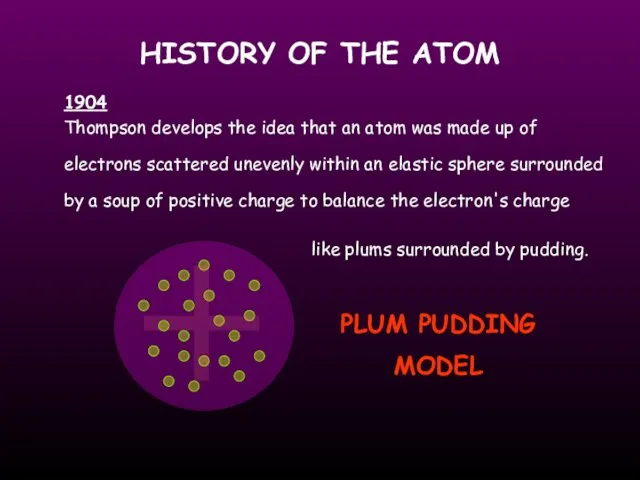
- 5. HISTORY OF THE ATOM Thompson develops the idea that an atom was made up of electrons
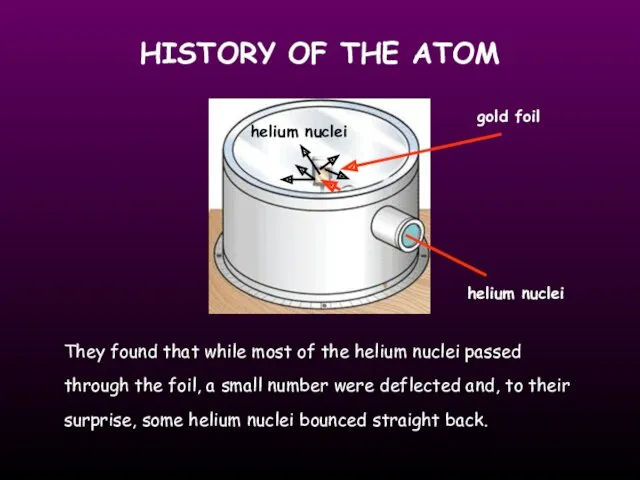
- 6. HISTORY OF THE ATOM 1910 Ernest Rutherford oversaw Geiger and Marsden carrying out his famous experiment.
- 7. HISTORY OF THE ATOM gold foil helium nuclei They found that while most of the helium
- 8. HISTORY OF THE ATOM Rutherford’s new evidence allowed him to propose a more detailed model with
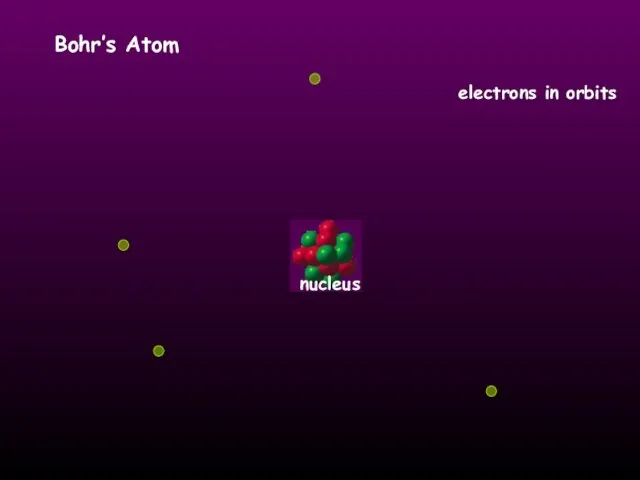
- 9. HISTORY OF THE ATOM 1913 Niels Bohr studied under Rutherford at the Victoria University in Manchester.
- 10. Bohr’s Atom electrons in orbits nucleus
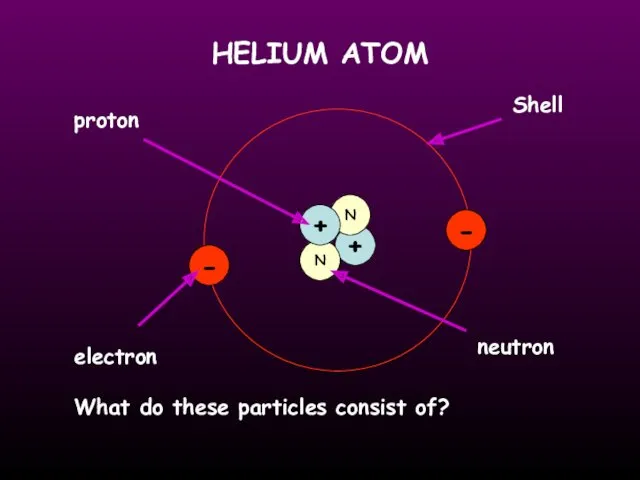
- 11. HELIUM ATOM + N N + - - proton electron neutron Shell What do these particles
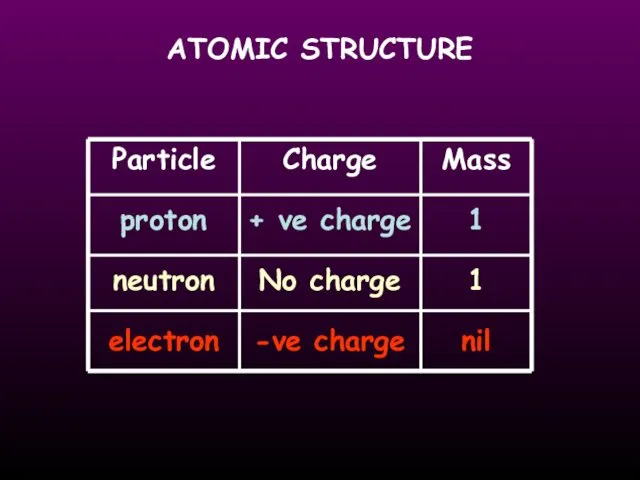
- 12. ATOMIC STRUCTURE Particle proton neutron electron Charge + ve charge -ve charge No charge 1 1
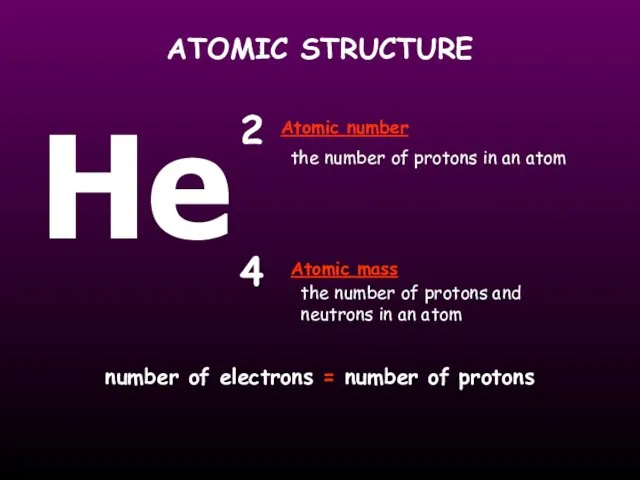
- 13. ATOMIC STRUCTURE the number of protons in an atom the number of protons and neutrons in
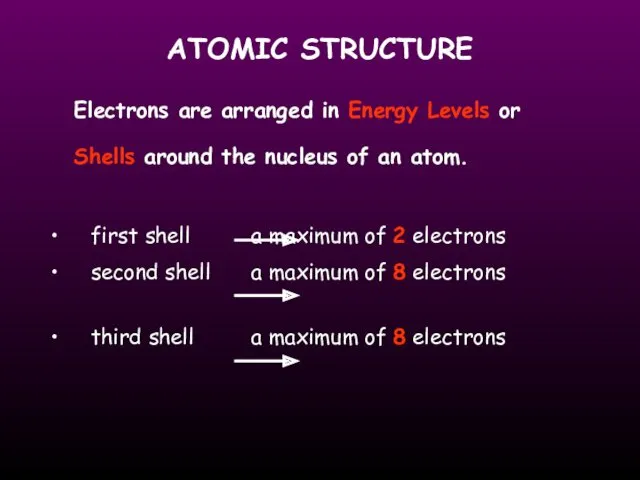
- 14. ATOMIC STRUCTURE Electrons are arranged in Energy Levels or Shells around the nucleus of an atom.
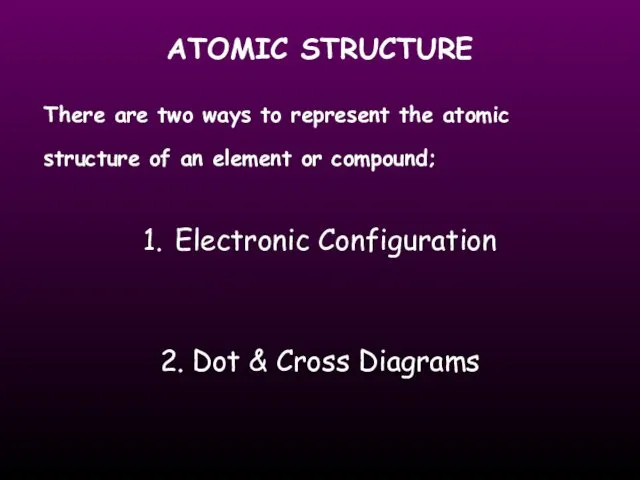
- 15. ATOMIC STRUCTURE There are two ways to represent the atomic structure of an element or compound;
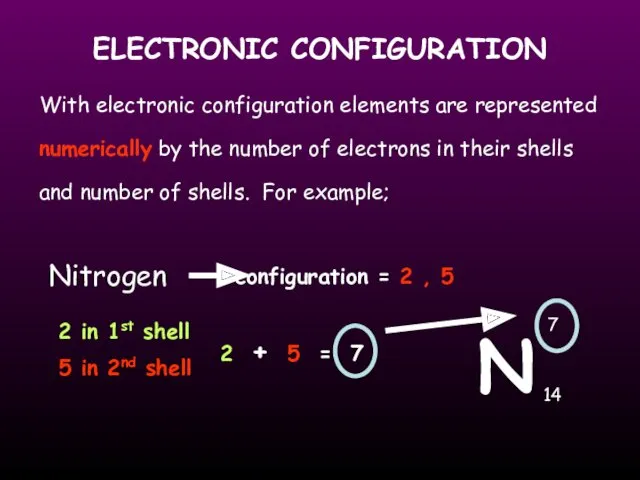
- 16. ELECTRONIC CONFIGURATION With electronic configuration elements are represented numerically by the number of electrons in their
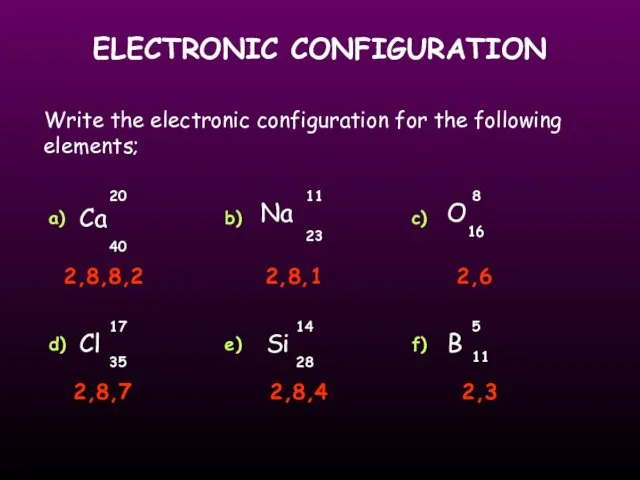
- 17. ELECTRONIC CONFIGURATION Write the electronic configuration for the following elements; Ca O Cl Si Na 20
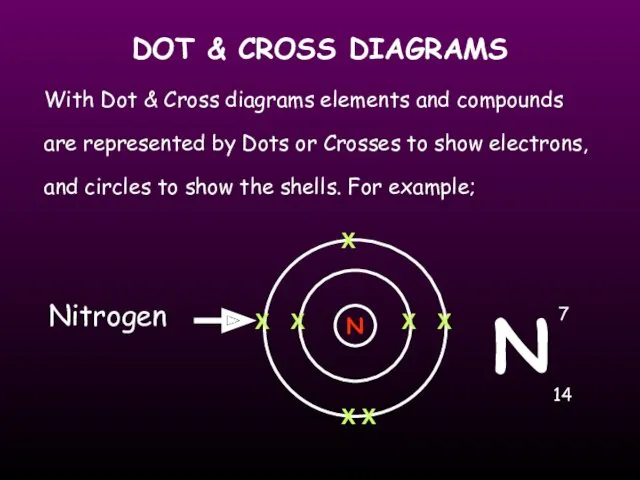
- 18. DOT & CROSS DIAGRAMS With Dot & Cross diagrams elements and compounds are represented by Dots
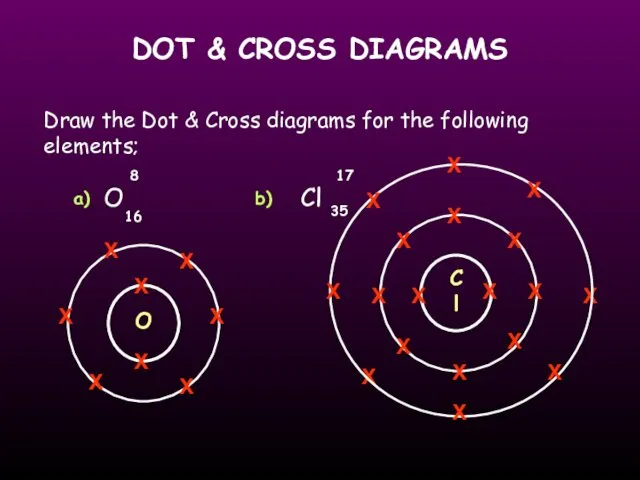
- 19. DOT & CROSS DIAGRAMS Draw the Dot & Cross diagrams for the following elements; O Cl
- 20. SUMMARY The Atomic Number of an atom = number of protons in the nucleus. The Atomic
- 22. Скачать презентацию







 Постоянный электрический ток
Постоянный электрический ток Электромагнетизм. Уравнения Максвелла. Лекция 4
Электромагнетизм. Уравнения Максвелла. Лекция 4 Электромагнитное поле. Электромагнитные волны
Электромагнитное поле. Электромагнитные волны Лабораторная работа по физике в 11 классе Наблюдение линейчатого и сплошного спектров
Лабораторная работа по физике в 11 классе Наблюдение линейчатого и сплошного спектров Датчики. Датчики крутящего момента. Датчики уровня
Датчики. Датчики крутящего момента. Датчики уровня Процедура разборки КПП Wet 8DCT (D8LF1)
Процедура разборки КПП Wet 8DCT (D8LF1) Unusual modes pf transport
Unusual modes pf transport Техническая термодинамика. Второй закон термодинамики. (Лекция 3)
Техническая термодинамика. Второй закон термодинамики. (Лекция 3) Простые механизмы. Работа. Мощность. Энергия
Простые механизмы. Работа. Мощность. Энергия Способы восстановления деталей
Способы восстановления деталей Магнитное поле. 11 класс
Магнитное поле. 11 класс Физические явления
Физические явления Электростатика. Электризация. Заряд. Взаимодействие зарядов. Закон Кулона
Электростатика. Электризация. Заряд. Взаимодействие зарядов. Закон Кулона Волновые процессы. Эффект Допплера. (Лекция 1)
Волновые процессы. Эффект Допплера. (Лекция 1) Электростатика. Тема 1. Электростатическое поле в вакууме
Электростатика. Тема 1. Электростатическое поле в вакууме Урок № 36 2 Промывочные жидкости
Урок № 36 2 Промывочные жидкости Линзы. Понятие о линзе
Линзы. Понятие о линзе Закони збереження в механіці
Закони збереження в механіці Электромагнитная индукция. Энергия магнитного поля. Лекция №11
Электромагнитная индукция. Энергия магнитного поля. Лекция №11 Модель атома. Опыт Резерфорда. Постулаты Бора
Модель атома. Опыт Резерфорда. Постулаты Бора Гидродинамика
Гидродинамика Охлаждение, нагревание тел конечных размеров. Нагрев параллелепипеда
Охлаждение, нагревание тел конечных размеров. Нагрев параллелепипеда Анализ сигналов
Анализ сигналов Физико – химические характеристики электротехнических материалов
Физико – химические характеристики электротехнических материалов Коробка скоростей
Коробка скоростей Электроразведка. Электромагнитные зондирования
Электроразведка. Электромагнитные зондирования Лекция 15. Тема: Закон Био-Савара - Лапласа
Лекция 15. Тема: Закон Био-Савара - Лапласа Предыстория радиотехники. Лекция 1
Предыстория радиотехники. Лекция 1