Содержание
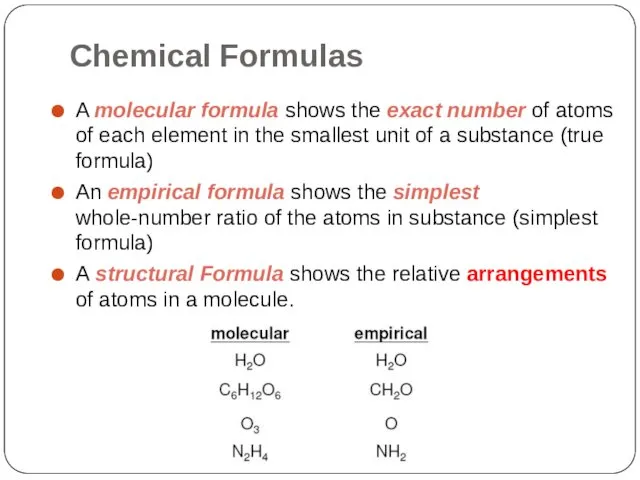
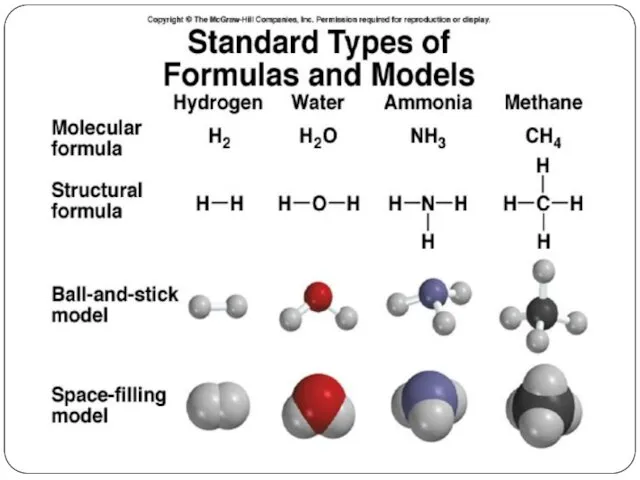
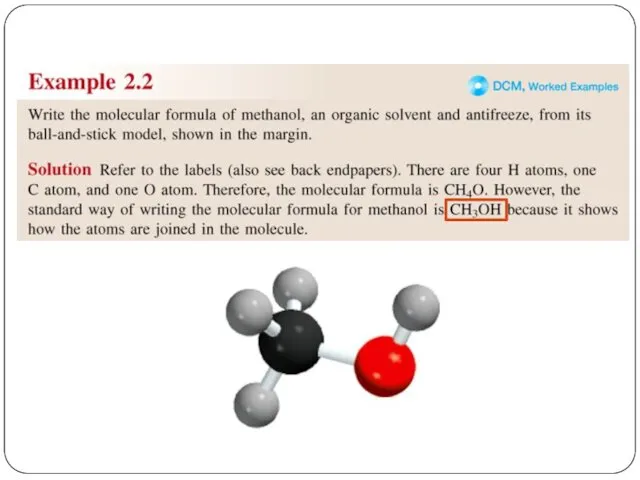
- 2. Chemical Formulas A molecular formula shows the exact number of atoms of each element in the
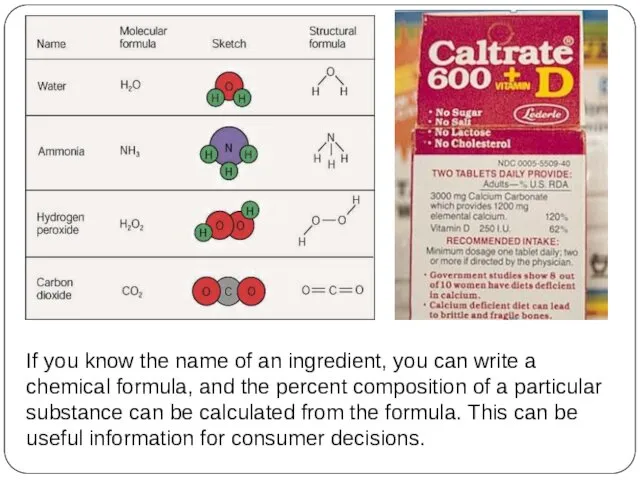
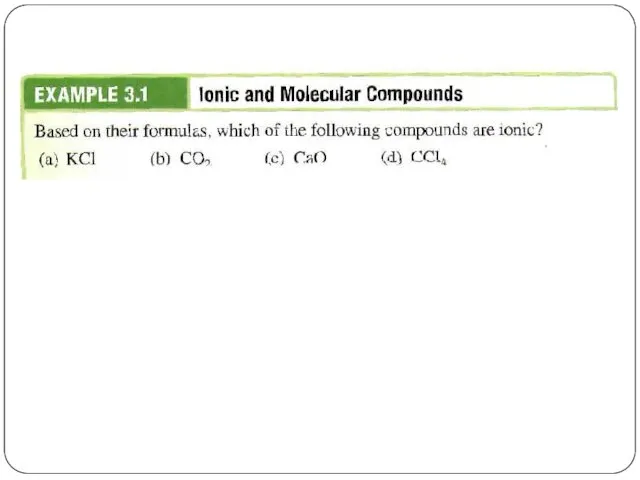
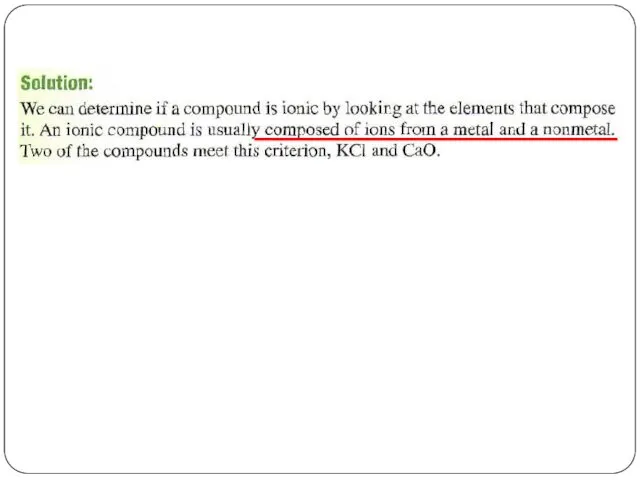
- 4. If you know the name of an ingredient, you can write a chemical formula, and the
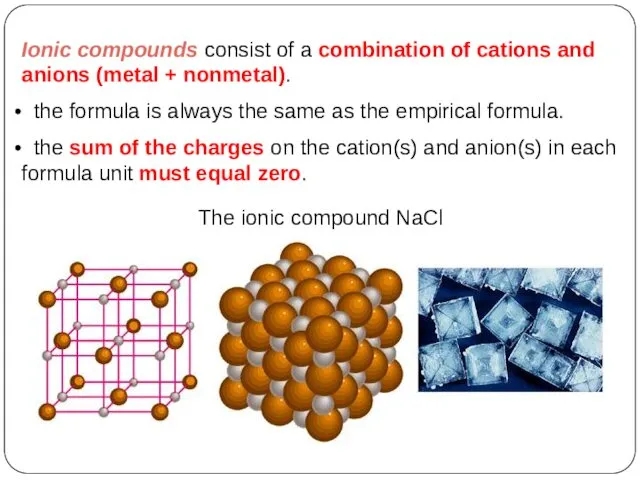
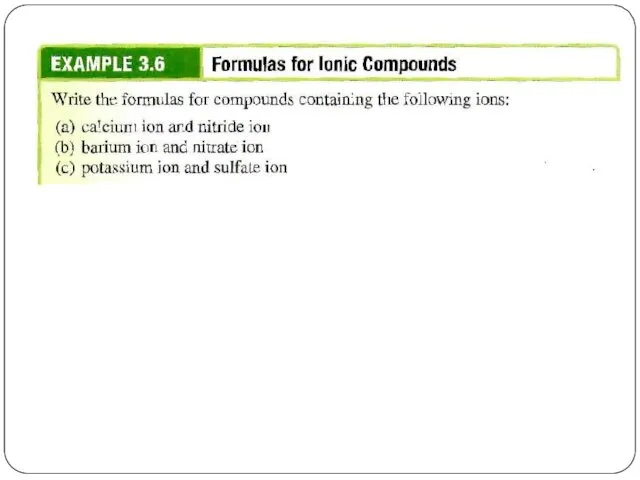
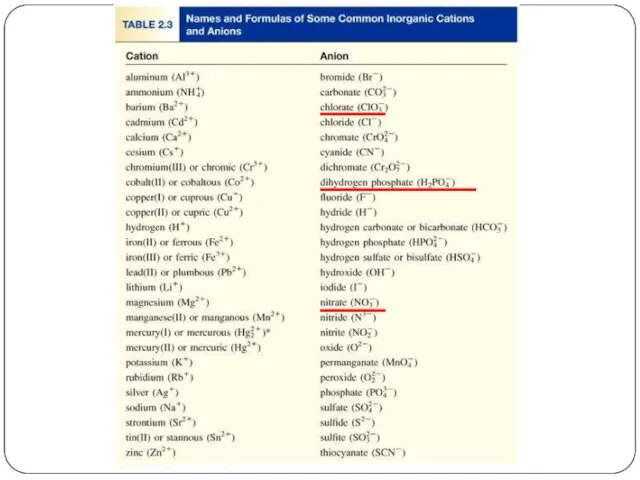
- 14. Ionic compounds consist of a combination of cations and anions (metal + nonmetal). the formula is
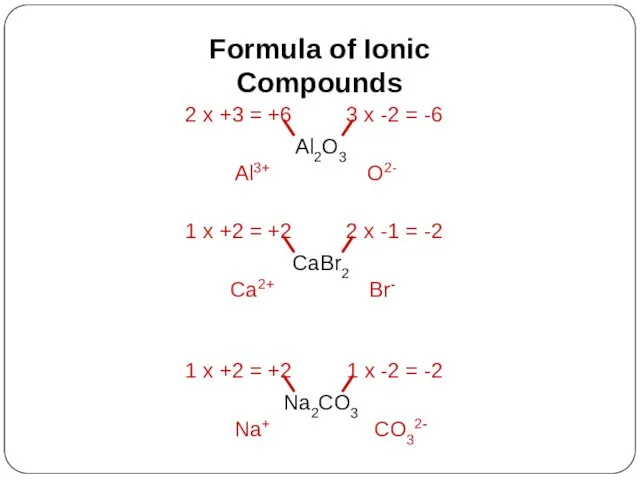
- 17. Formula of Ionic Compounds Al2O3 Al3+ O2- CaBr2 Ca2+ Br- Na2CO3 Na+ CO32-
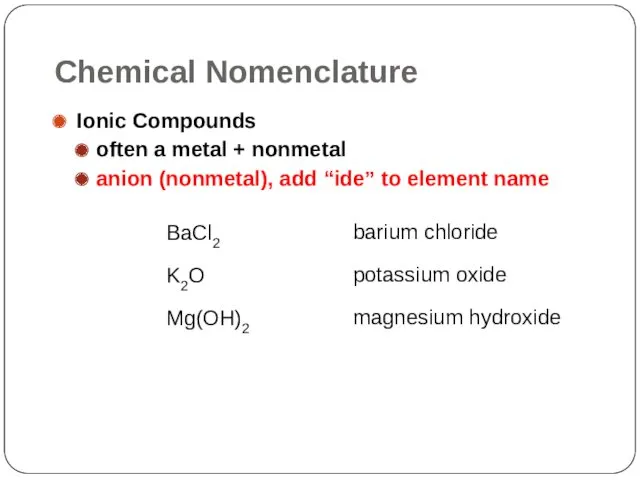
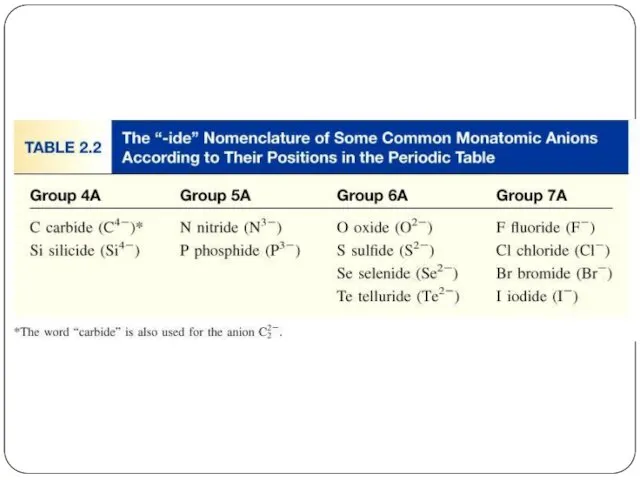
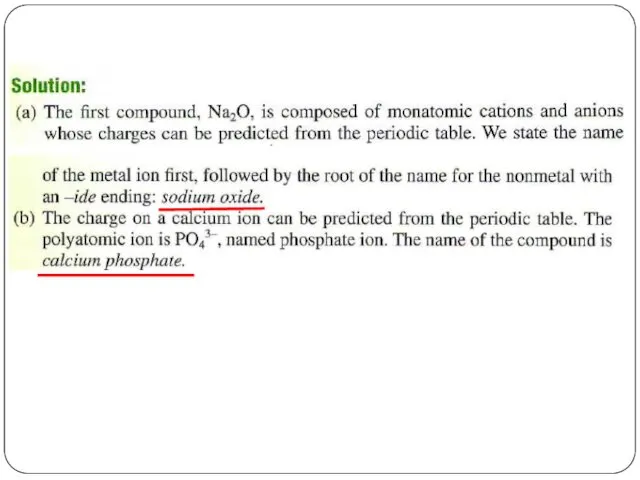
- 22. Chemical Nomenclature Ionic Compounds often a metal + nonmetal anion (nonmetal), add “ide” to element name
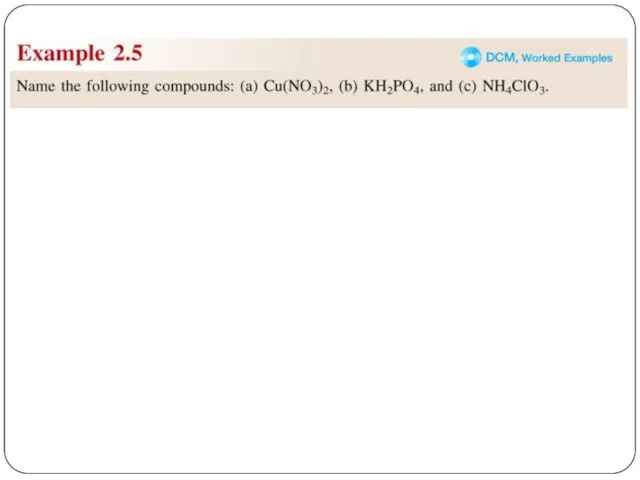
- 23. Transition metal ionic compounds indicate charge on metal with Roman numerals羅馬數字 FeCl2 2 Cl- -2, so
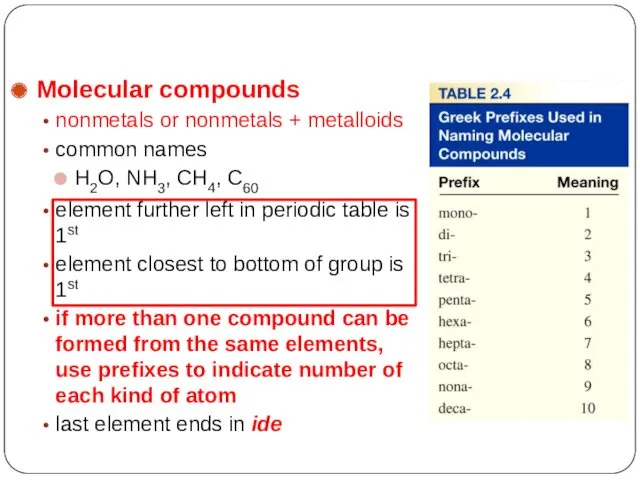
- 31. Molecular compounds nonmetals or nonmetals + metalloids common names H2O, NH3, CH4, C60 element further left
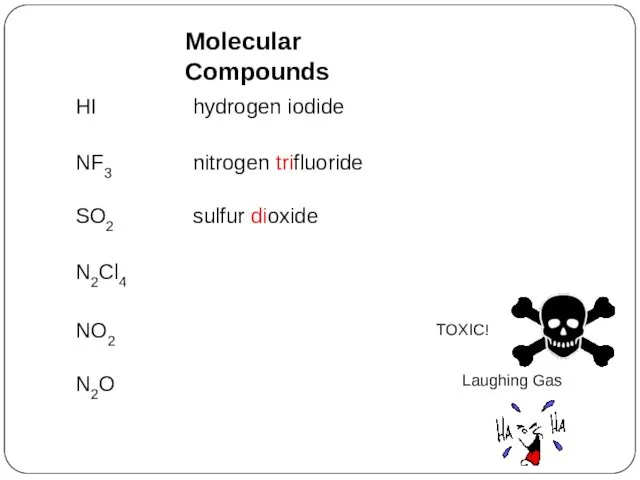
- 32. HI hydrogen iodide NF3 nitrogen trifluoride SO2 sulfur dioxide N2Cl4 NO2 N2O Molecular Compounds
- 35. Скачать презентацию











 Основні закони хімії.Класи та номенклатура неорганічних сполук
Основні закони хімії.Класи та номенклатура неорганічних сполук Общие вопросы аналитической химии. Химические методы обнаружения неорганических веществ
Общие вопросы аналитической химии. Химические методы обнаружения неорганических веществ Элементы IV А - группы
Элементы IV А - группы Халькогены
Халькогены Складні білки
Складні білки Периодический закон и периодическая система химических элементов Д.И. Менделеева
Периодический закон и периодическая система химических элементов Д.И. Менделеева Химические и физические явления в жизни человека
Химические и физические явления в жизни человека Обучение в сотрудничестве на уроках химии
Обучение в сотрудничестве на уроках химии Спирты. Понятия о предельных одноатомных спиртах. Химические свойства этанола и его применение
Спирты. Понятия о предельных одноатомных спиртах. Химические свойства этанола и его применение Йони. Йонний зв’язок, його утворення
Йони. Йонний зв’язок, його утворення Оценка химической обстановки при авариях на химически опасных объектах. Расчет
Оценка химической обстановки при авариях на химически опасных объектах. Расчет Значение пищи и ее состав
Значение пищи и ее состав Методические подходы к решению химических задач. Задание 34
Методические подходы к решению химических задач. Задание 34 Соединения железа
Соединения железа Нұсқа талдау
Нұсқа талдау Основы фармацевтической химии
Основы фармацевтической химии Водород. Н2
Водород. Н2 Гидроксид аммония
Гидроксид аммония Кислоты. Состав кислот
Кислоты. Состав кислот Preparation for COP
Preparation for COP ВОДОРОД
ВОДОРОД Получение и применение алканов
Получение и применение алканов Аммиак
Аммиак Объемная доля компонента газовой смеси
Объемная доля компонента газовой смеси Свойства фосфора
Свойства фосфора Химический состав и физические свойства продовольственных товаров
Химический состав и физические свойства продовольственных товаров Фенолдар, аминдер, альдегидтер
Фенолдар, аминдер, альдегидтер Железо. Нахождение в природе. Свойства железа
Железо. Нахождение в природе. Свойства железа