Содержание
- 2. Name the compounds: CuSO4 NaOH HNO3 NH4NO3 Ca(OH)2 H2SiO3 H3BO3 KMnO4 Na[Al(OH)4] NaH2PO4 Na2HPO4 PbI2 HNO2
- 3. General Properties of Aqueous Solutions Solution - a homogeneous mixture Solute: the component that is dissolved
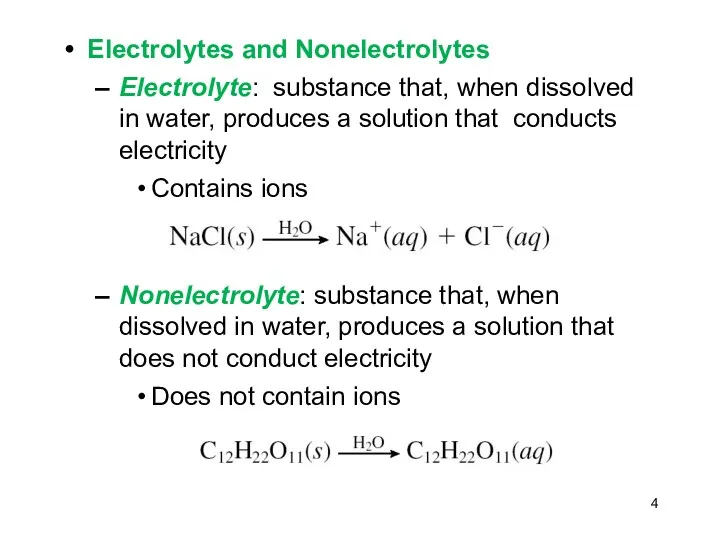
- 4. Electrolytes and Nonelectrolytes Electrolyte: substance that, when dissolved in water, produces a solution that conducts electricity
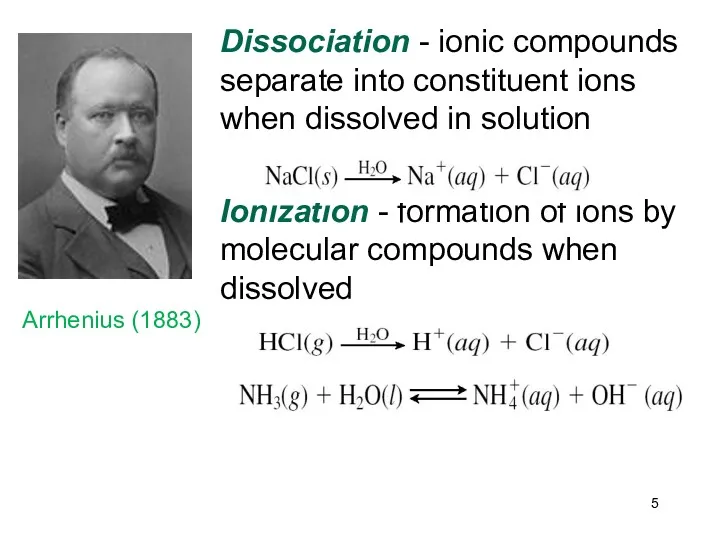
- 5. Dissociation - ionic compounds separate into constituent ions when dissolved in solution Ionization - formation of
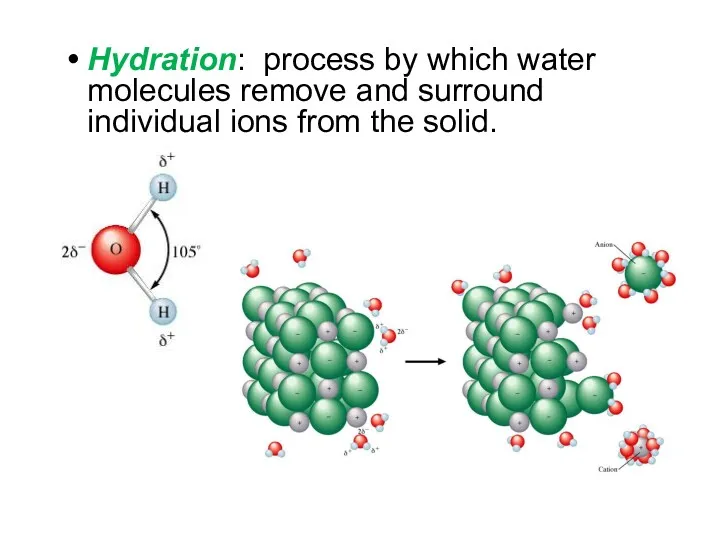
- 6. Hydration: process by which water molecules remove and surround individual ions from the solid.
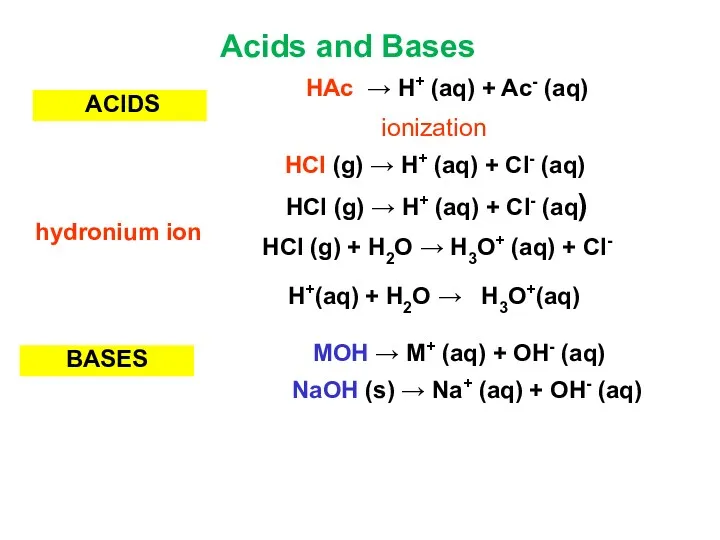
- 7. ACIDS BASES NaOH (s) → Na+ (aq) + OH- (aq) MOH → M+ (aq) + OH-
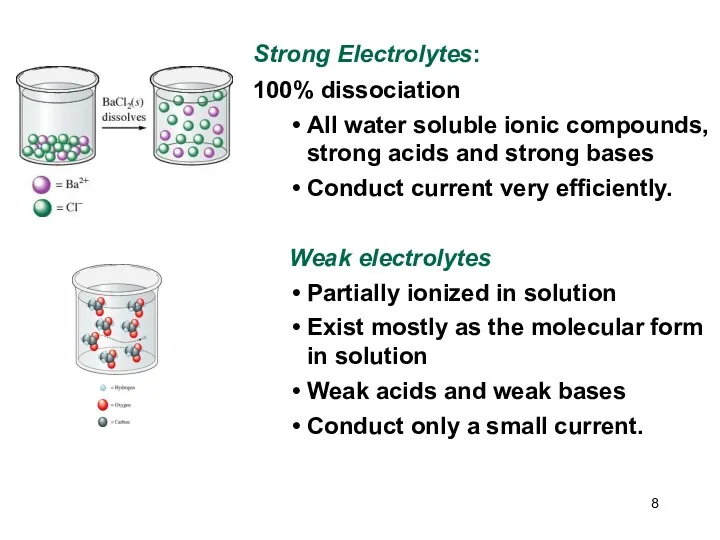
- 8. Strong Electrolytes: 100% dissociation All water soluble ionic compounds, strong acids and strong bases Conduct current
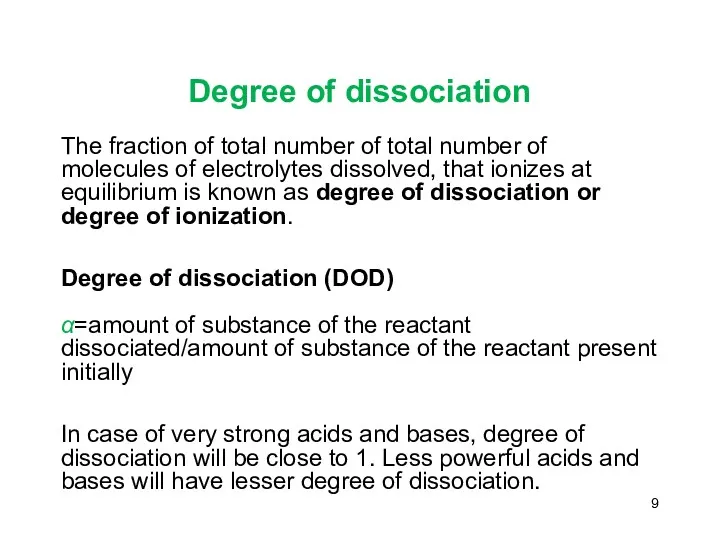
- 9. Degree of dissociation The fraction of total number of total number of molecules of electrolytes dissolved,
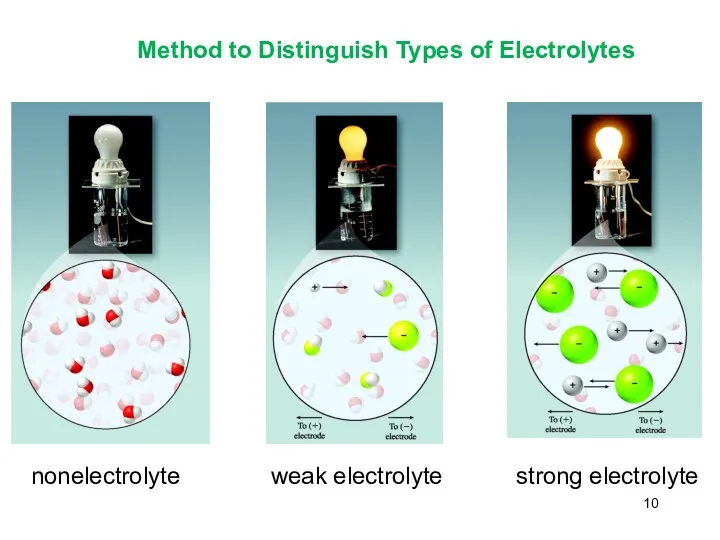
- 10. Method to Distinguish Types of Electrolytes nonelectrolyte weak electrolyte strong electrolyte
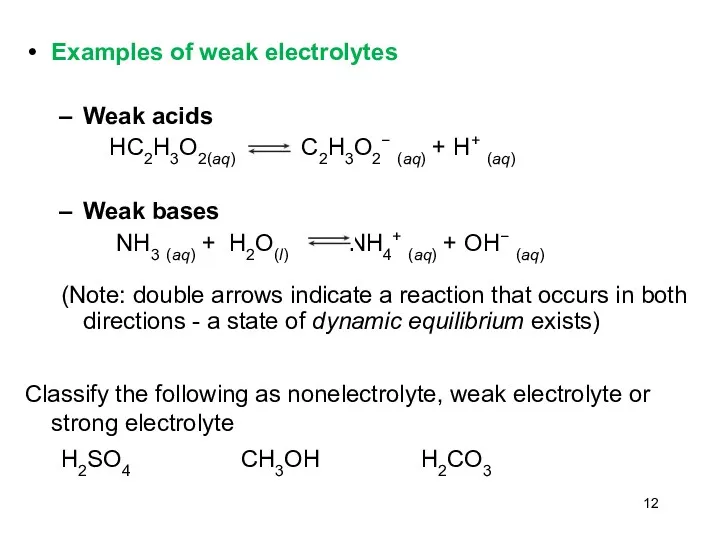
- 12. Examples of weak electrolytes Weak acids HC2H3O2(aq) C2H3O2− (aq) + H+ (aq) Weak bases NH3 (aq)
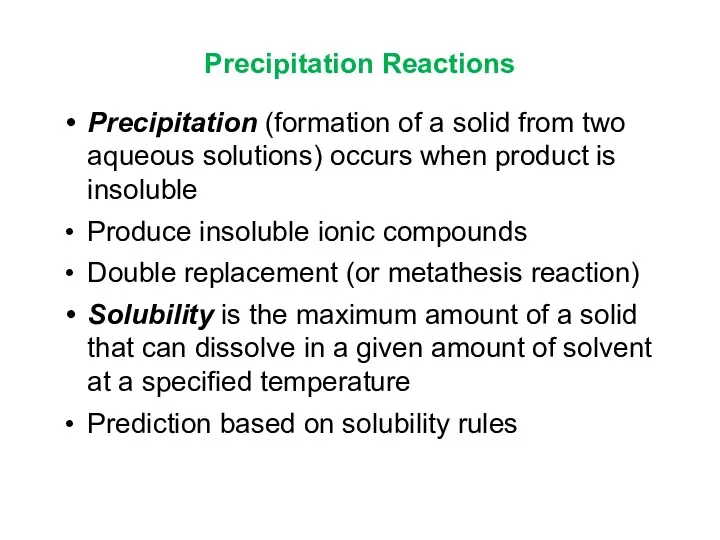
- 13. Precipitation Reactions Precipitation (formation of a solid from two aqueous solutions) occurs when product is insoluble
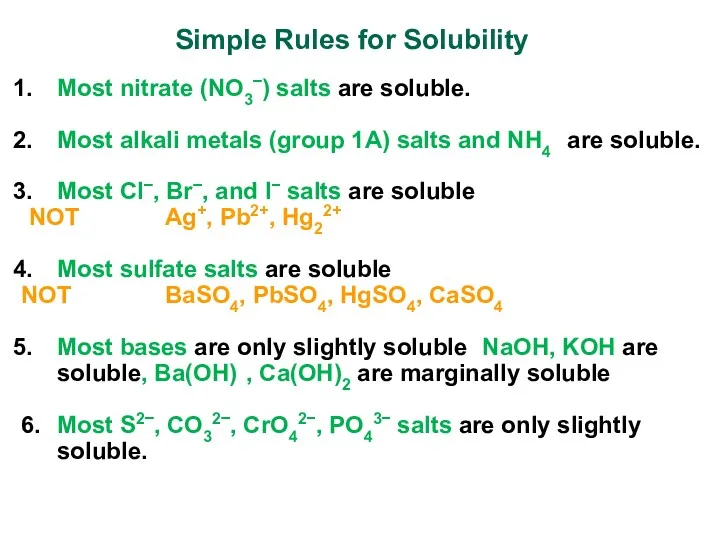
- 14. Simple Rules for Solubility Most nitrate (NO3−) salts are soluble. Most alkali metals (group 1A) salts
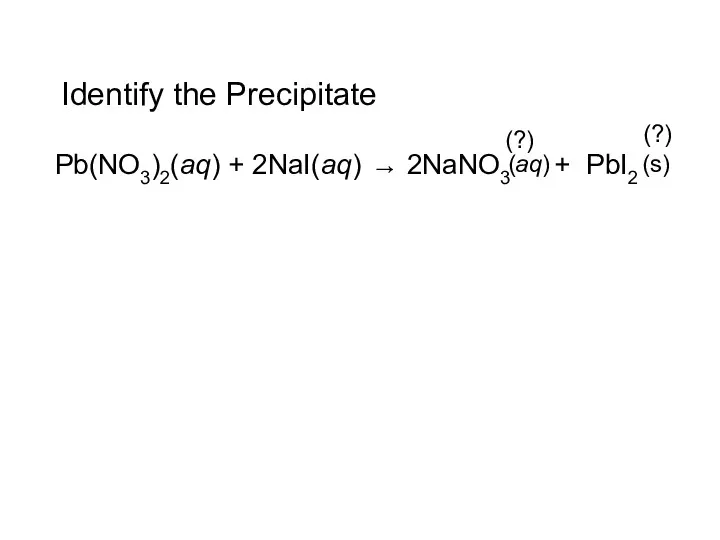
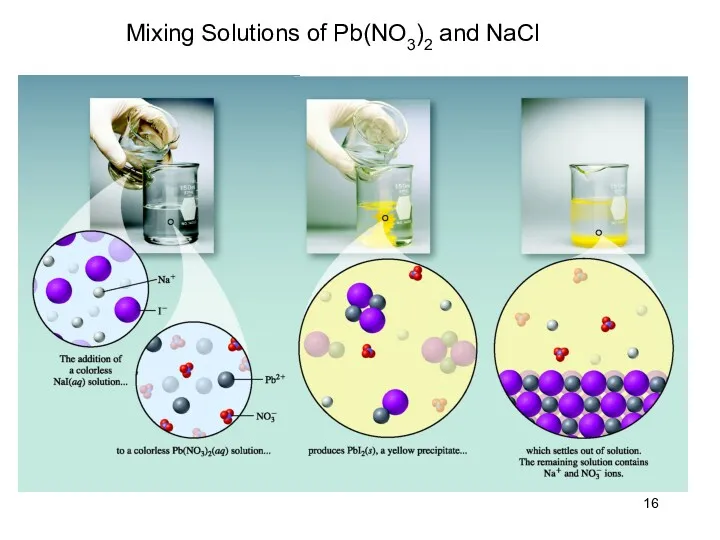
- 15. Identify the Precipitate Pb(NO3)2(aq) + 2NaI(aq) → 2NaNO3 + PbI2 (s) (aq) (?) (?)
- 16. Mixing Solutions of Pb(NO3)2 and NaCl
- 17. Molecular equation: shows all compounds represented by their chemical formulas Ionic equation: shows all strong electrolytes
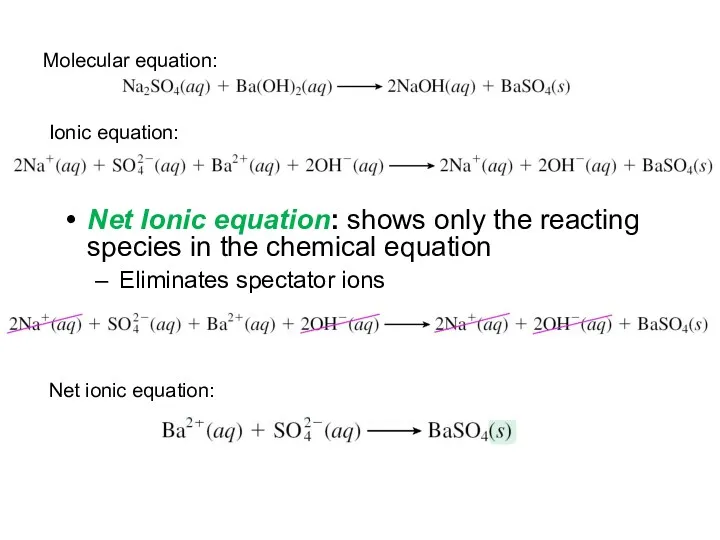
- 18. Net Ionic equation: shows only the reacting species in the chemical equation Eliminates spectator ions Molecular
- 19. Steps in writing a net ionic equation Write the balanced molecular equation. Predict products by exchanging
- 20. Aqueous solutions of silver nitrate and sodium sulfate are mixed. Write the net ionic reaction. 2AgNO3(aq)+Na2SO4(aq)
- 21. Aqueous Reactions and Chemical Analysis Types of quantitative analysis Gravimetric analysis (mass analysis) Example: precipitation reaction
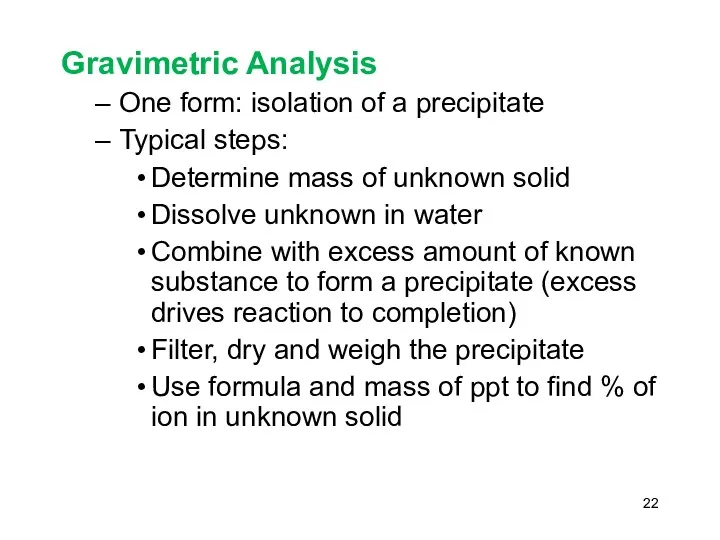
- 22. Gravimetric Analysis One form: isolation of a precipitate Typical steps: Determine mass of unknown solid Dissolve
- 23. A 0.825 g sample of an ionic compound containing chloride ions and an unknown metal is
- 24. Steps in solution: Find the % of Cl in AgCl Multiply the % of Cl by
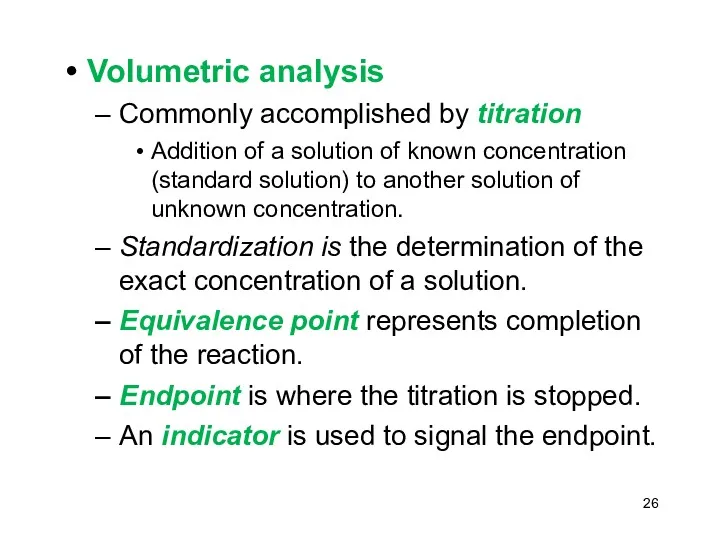
- 26. Volumetric analysis Commonly accomplished by titration Addition of a solution of known concentration (standard solution) to
- 27. Apparatus for Titration
- 28. A student measured exactly 15.0 mL of an unknown monoprotic acidic solution and placed in an
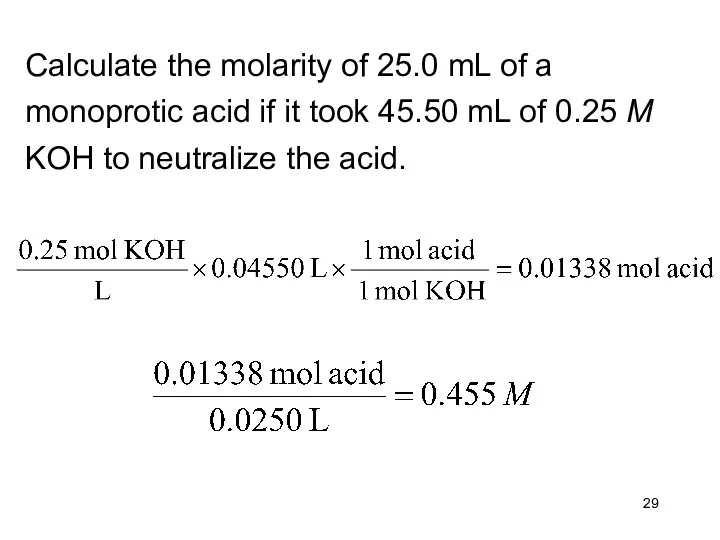
- 29. Calculate the molarity of 25.0 mL of a monoprotic acid if it took 45.50 mL of
- 31. Скачать презентацию
![Name the compounds: CuSO4 NaOH HNO3 NH4NO3 Ca(OH)2 H2SiO3 H3BO3 KMnO4 Na[Al(OH)4] NaH2PO4 Na2HPO4 PbI2 HNO2](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/213916/slide-1.jpg)

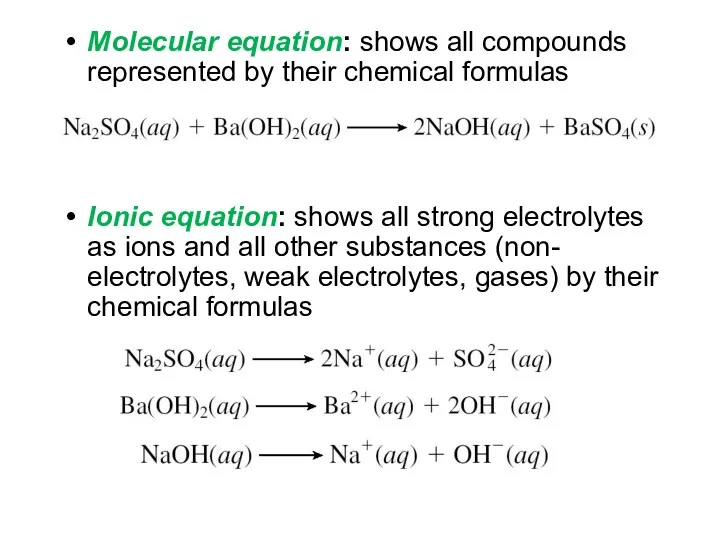







 Начало нанотехнологической эры. Фуллерены
Начало нанотехнологической эры. Фуллерены Закон триад. Открытие периодического закона
Закон триад. Открытие периодического закона Металлическая связь. Агрегатные состояния вещества
Металлическая связь. Агрегатные состояния вещества Основания. Получение и химические свойства
Основания. Получение и химические свойства Азот. Соединения азота
Азот. Соединения азота Окисно-відновні реакції у природі і промисловості
Окисно-відновні реакції у природі і промисловості Обмен липидов
Обмен липидов Типы химических соединений, номенклатура, свойства
Типы химических соединений, номенклатура, свойства Застосування алканів
Застосування алканів ЕГЭ 2017. Подготовка к вопросу № 26
ЕГЭ 2017. Подготовка к вопросу № 26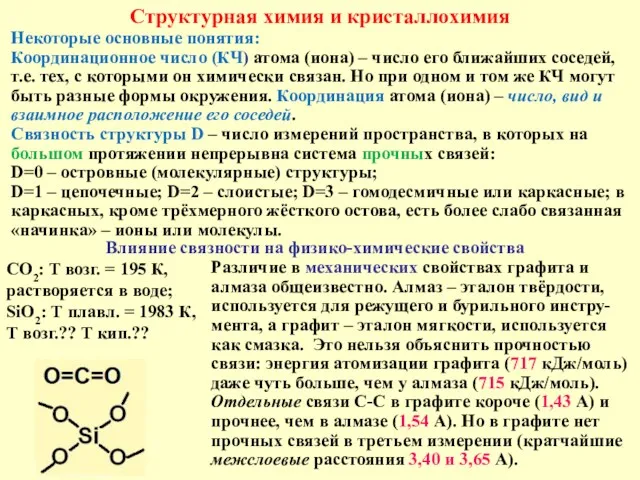 Структурная химия и кристаллохимия
Структурная химия и кристаллохимия Жиры и мыло
Жиры и мыло Ароматты комирсутектер Бензол
Ароматты комирсутектер Бензол Основания и кислоты. Химические и физические свойства
Основания и кислоты. Химические и физические свойства Гетероциклические соединения. Шестичленные гетероциклы с одним гетероатомом
Гетероциклические соединения. Шестичленные гетероциклы с одним гетероатомом Превращения веществ. Роль химии в жизни человека
Превращения веществ. Роль химии в жизни человека Минеральные вещества
Минеральные вещества Химия и производство
Химия и производство Theories of acids and bases. Ionic equilibria in electrolyte solutions. Buffer solutions (topic 3.4)
Theories of acids and bases. Ionic equilibria in electrolyte solutions. Buffer solutions (topic 3.4) Материаловедение. Атомно-кристаллическое строение металлов и сплавов. (Тема 3)
Материаловедение. Атомно-кристаллическое строение металлов и сплавов. (Тема 3) Синтетические топлива
Синтетические топлива Классификация химических реакций, протекающих с изменением состава веществ
Классификация химических реакций, протекающих с изменением состава веществ Игровая программа по химии Самый умный. Химические свойства оксидов, оснований, кислот и солей
Игровая программа по химии Самый умный. Химические свойства оксидов, оснований, кислот и солей Энергетика химических процессов
Энергетика химических процессов Какими свойствами обладает воздух. Исследовательская работа
Какими свойствами обладает воздух. Исследовательская работа Хімічні властивості карбонових кислот
Хімічні властивості карбонових кислот Полівінілхлорид
Полівінілхлорид Реакции окисления и восстановления органических соединений
Реакции окисления и восстановления органических соединений