Содержание
- 2. Physical properties Hydrogen chloride is a gas with an irritating odour. An aqueous solution of HCl
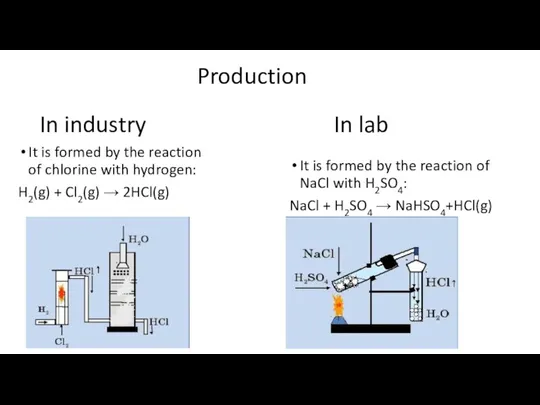
- 3. Production It is formed by the reaction of chlorine with hydrogen: H2(g) + Cl2(g) → 2HCl(g)
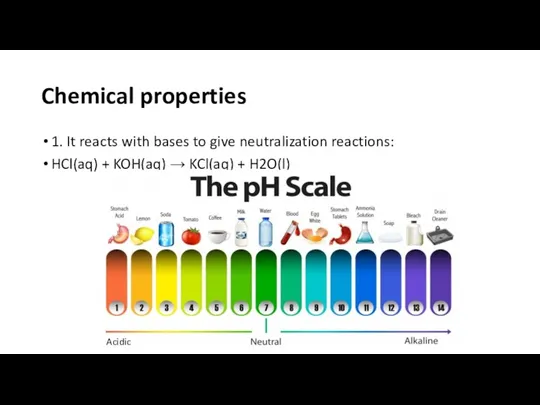
- 4. Chemical properties 1. It reacts with bases to give neutralization reactions: HCl(aq) + KOH(aq) → KCl(aq)
- 5. 2. It reacts with AgNO3, and a white precipitate is formed: HCl(aq) + AgNO3(aq) → AgCl(s)
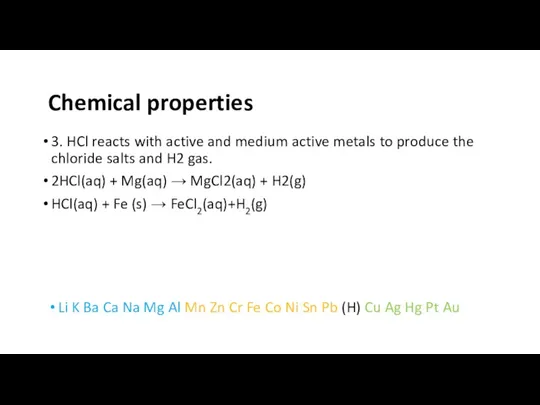
- 6. 3. HCl reacts with active and medium active metals to produce the chloride salts and H2
- 7. 4. It reacts with oxidizing agents such as KMnO4 to produce Cl2(g): 16HCl(aq) + 2KMnO4(aq) →
- 8. How to solve problems? (5 steps) 1. Write down the reaction 2. Balance it 3. Find
- 9. Problems What is the number of moles of Cl2 required to produce 146 g HCl?
- 10. Problems A 30 g sample of iron reacts with 200 g of 14.6% HCl solution by
- 12. Скачать презентацию






 Углеводы (особенности строения, реакционной способности и методы синтеза альдегидо- и кетоспиртов)
Углеводы (особенности строения, реакционной способности и методы синтеза альдегидо- и кетоспиртов) Атом – сложная частица
Атом – сложная частица Органика вокруг нас
Органика вокруг нас Молекулалық орбиталдың негіздері ТФП 315
Молекулалық орбиталдың негіздері ТФП 315 Получение и применение алканов
Получение и применение алканов Неметаллы. Азот
Неметаллы. Азот Катализ металлами. Лекция 3
Катализ металлами. Лекция 3 Загальні відомості про хімічну зброю. Кодування засобів застосування хімічних боєприпасів
Загальні відомості про хімічну зброю. Кодування засобів застосування хімічних боєприпасів Органічні сполуки (9 клас)
Органічні сполуки (9 клас) Кислоты
Кислоты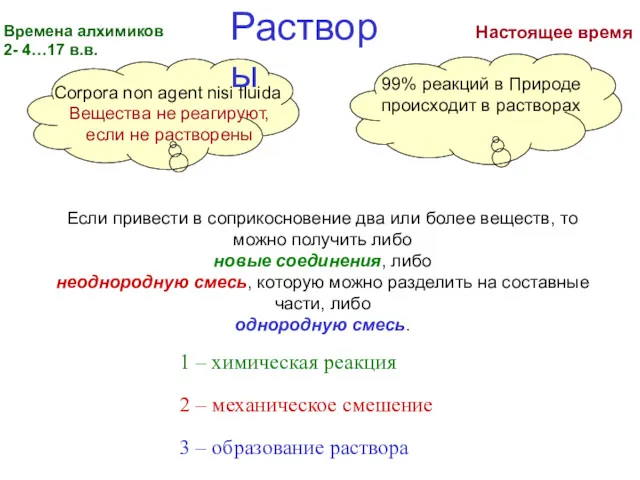 Времена алхимиков
Времена алхимиков Характеристика химического элемента по кислотно-основным свойствам образуемых им соединений. Амфотерные оксиды и гидроксиды
Характеристика химического элемента по кислотно-основным свойствам образуемых им соединений. Амфотерные оксиды и гидроксиды Язык химии
Язык химии Предмет аналитической химии и ее основные понятия
Предмет аналитической химии и ее основные понятия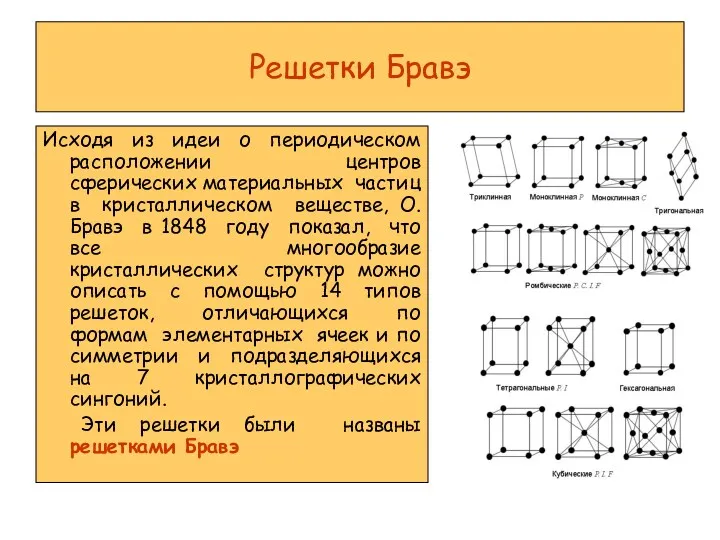 Решетки Бравэ
Решетки Бравэ Приемы обращения с лабораторным оборудованием
Приемы обращения с лабораторным оборудованием Спектральные методы: инфракрасная спектроскопия. Люминесцентный анализ
Спектральные методы: инфракрасная спектроскопия. Люминесцентный анализ Водород
Водород Массовая доля вещества в растворе
Массовая доля вещества в растворе Химический анализ почв. Понятия и показатели
Химический анализ почв. Понятия и показатели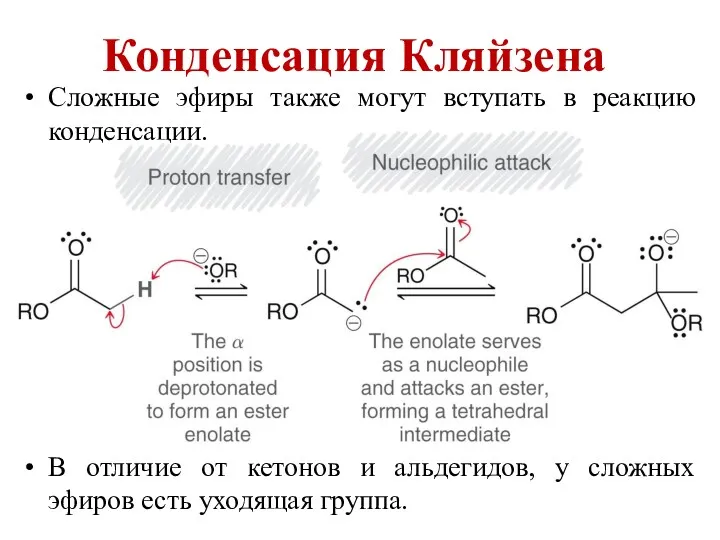 Конденсация Кляйзена
Конденсация Кляйзена Волшебные кристалы
Волшебные кристалы Решение задачи №4. Старость - на радость. Команда Карбораны
Решение задачи №4. Старость - на радость. Команда Карбораны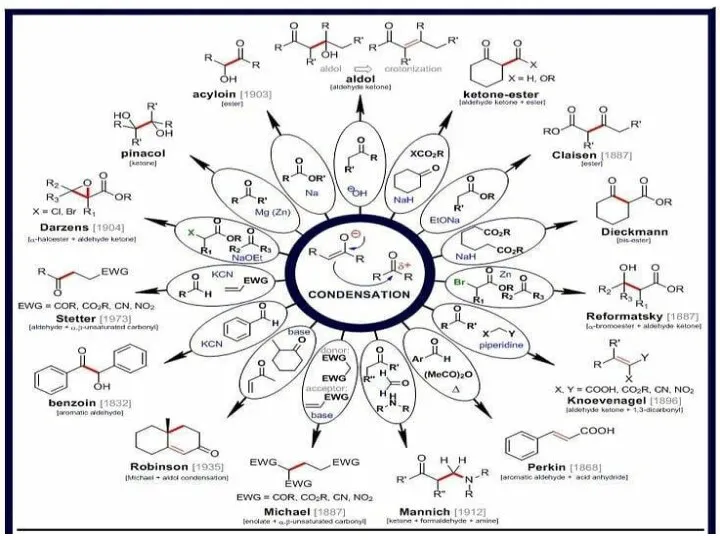 Альдольные реакции енолятов
Альдольные реакции енолятов От алхимии к химии
От алхимии к химии Химический элемент йод
Химический элемент йод Скорость химических реакций. Факторы, влияющие на скорость химической реакции (лекция № 5)
Скорость химических реакций. Факторы, влияющие на скорость химической реакции (лекция № 5) Calcium and magnesium. Formation of calcareous.water hardness
Calcium and magnesium. Formation of calcareous.water hardness