Содержание
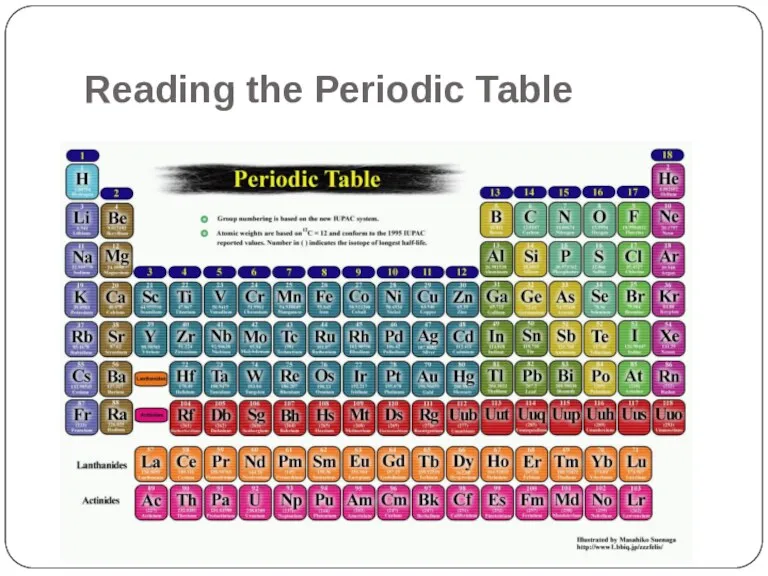
- 2. Reading the Periodic Table
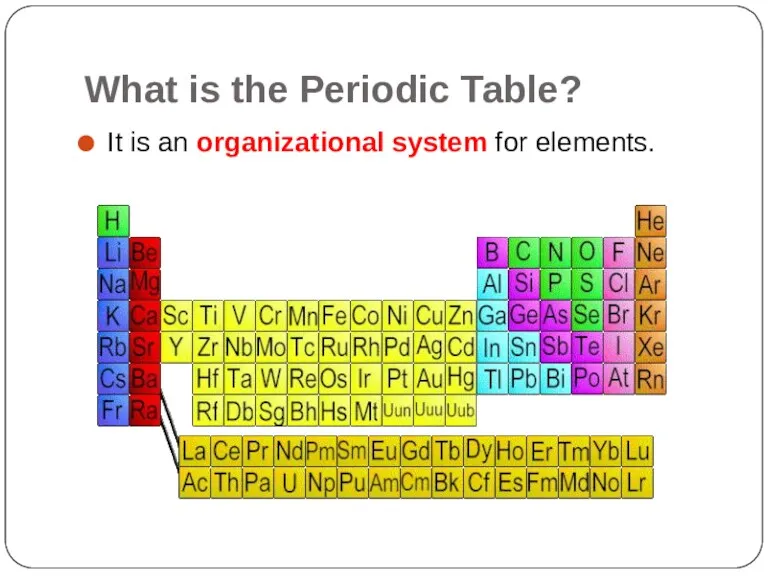
- 3. What is the Periodic Table? It is an organizational system for elements.
- 4. Who created it? The quest for a systematic arrangement of the elements started with the discovery
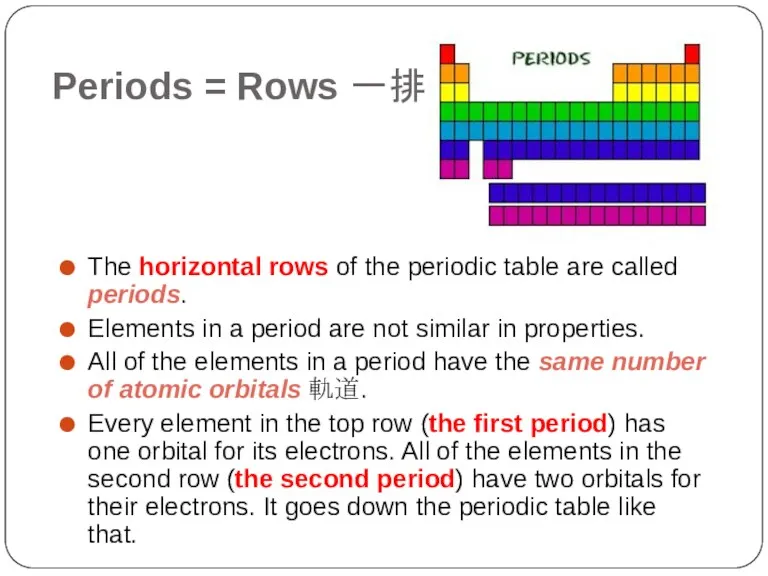
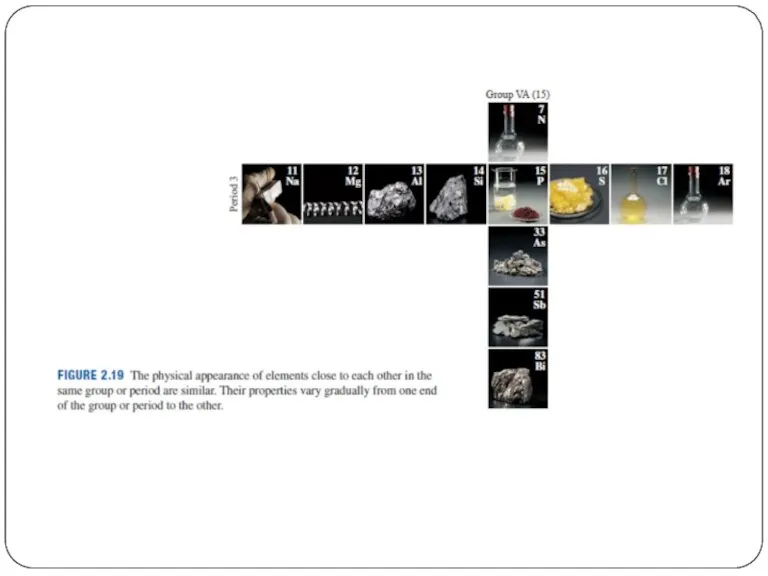
- 5. Periods = Rows 一排 The horizontal rows of the periodic table are called periods. Elements in
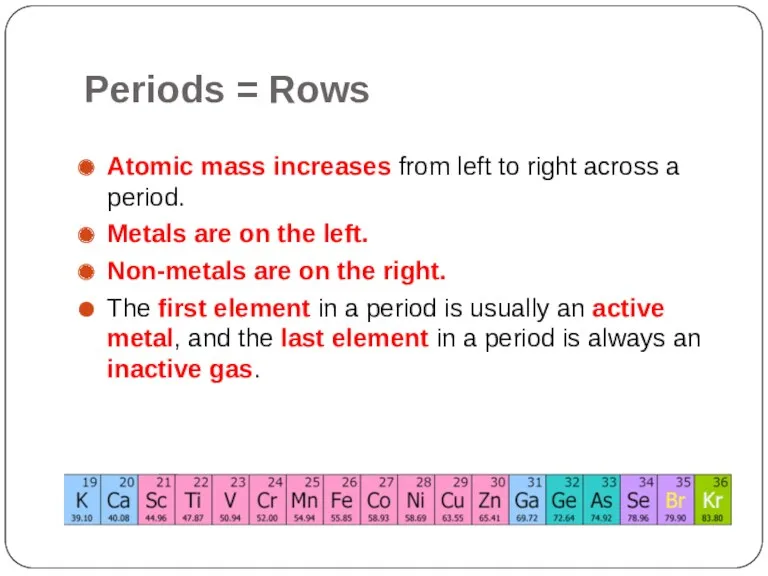
- 6. Periods = Rows Atomic mass increases from left to right across a period. Metals are on
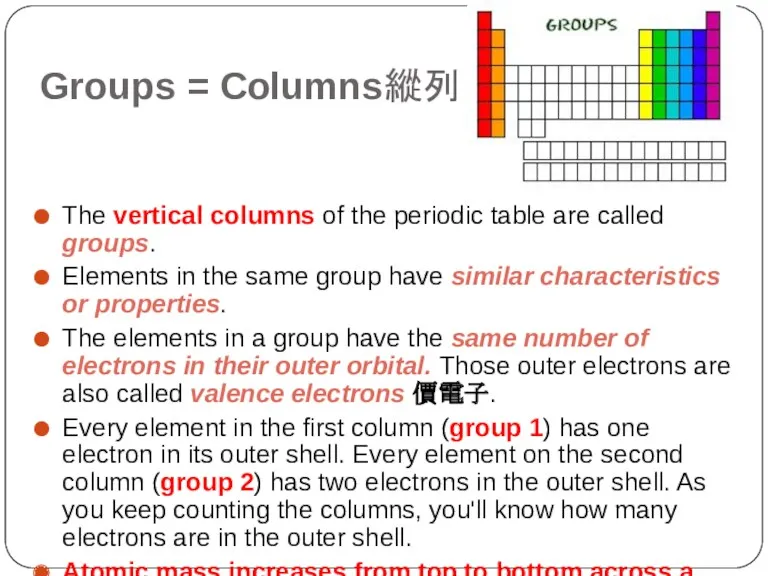
- 7. Groups = Columns縱列 The vertical columns of the periodic table are called groups. Elements in the
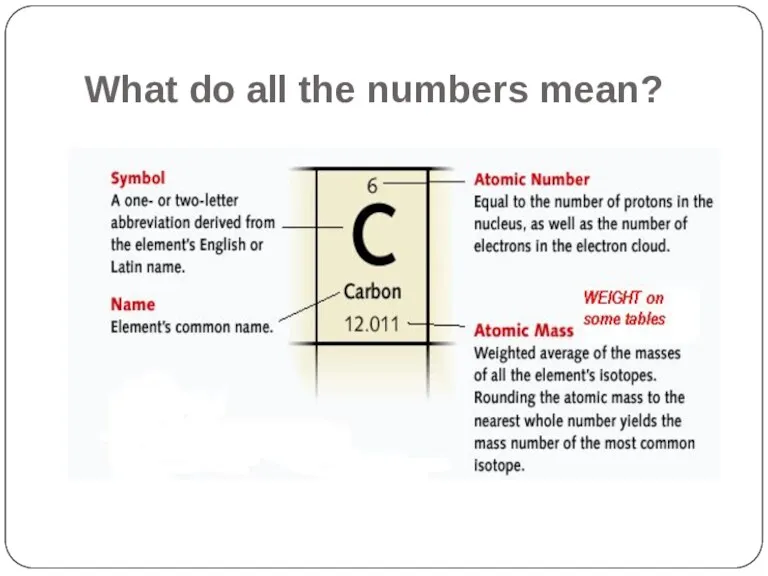
- 8. What do all the numbers mean?
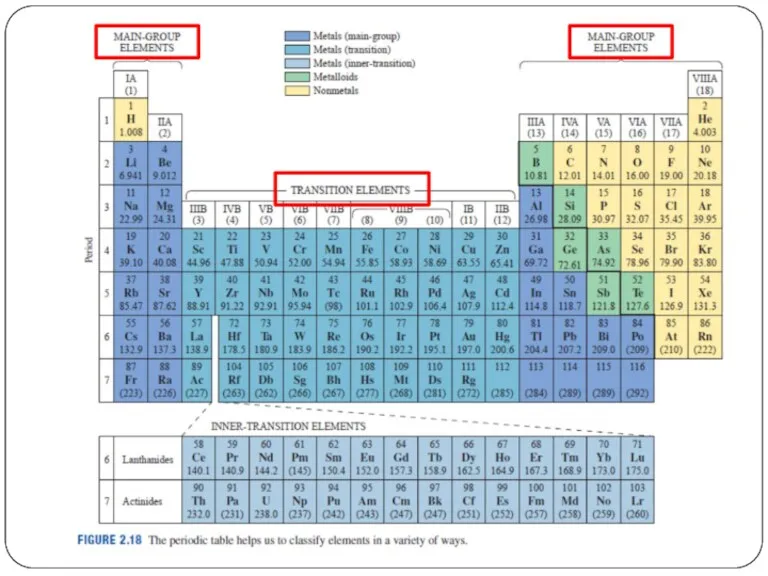
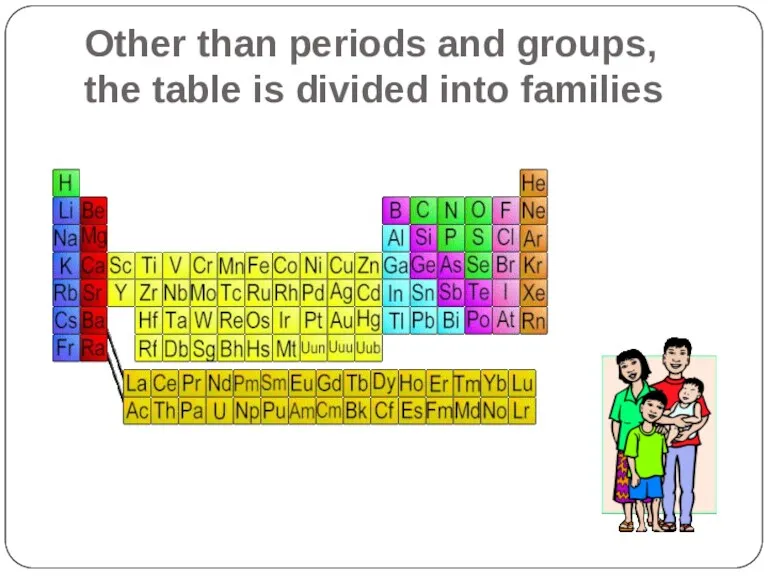
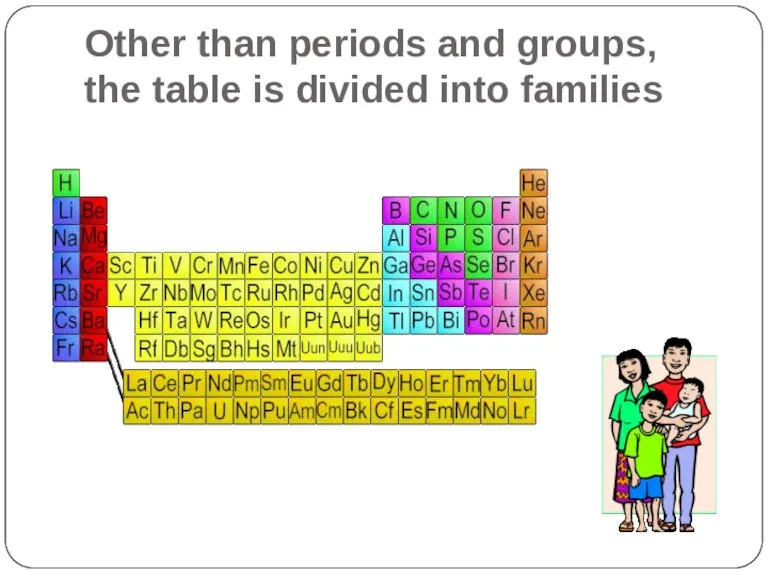
- 11. Other than periods and groups, the table is divided into families
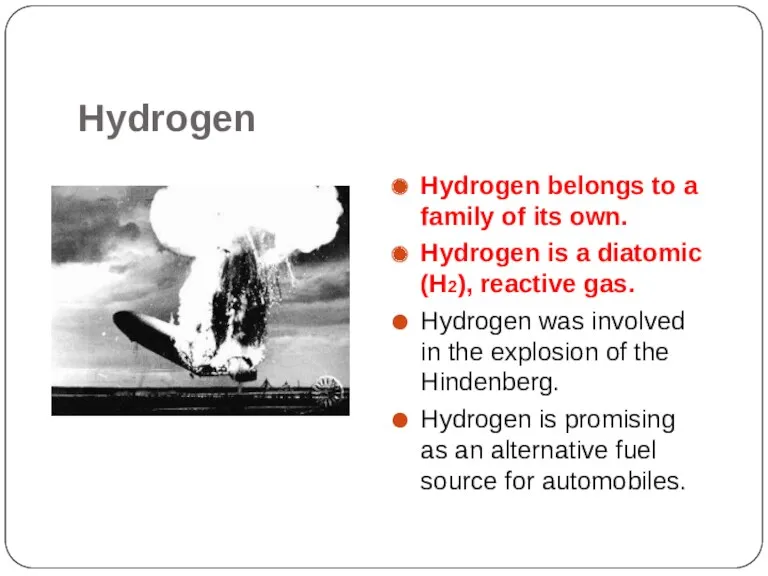
- 12. Hydrogen Hydrogen belongs to a family of its own. Hydrogen is a diatomic (H2), reactive gas.
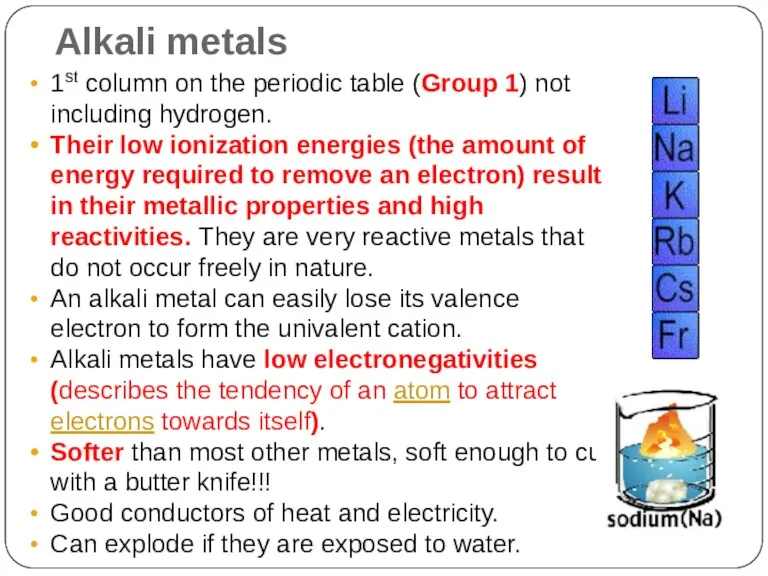
- 13. Alkali metals 1st column on the periodic table (Group 1) not including hydrogen. Their low ionization
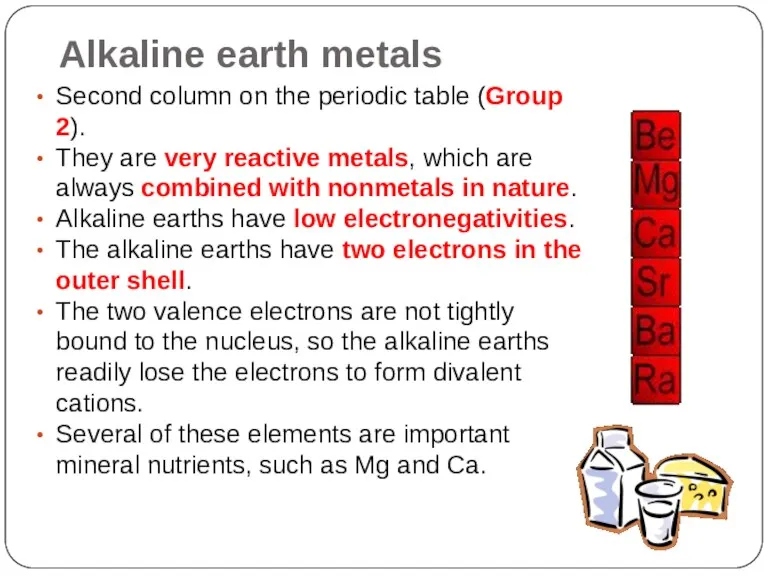
- 14. Alkaline earth metals Second column on the periodic table (Group 2). They are very reactive metals,
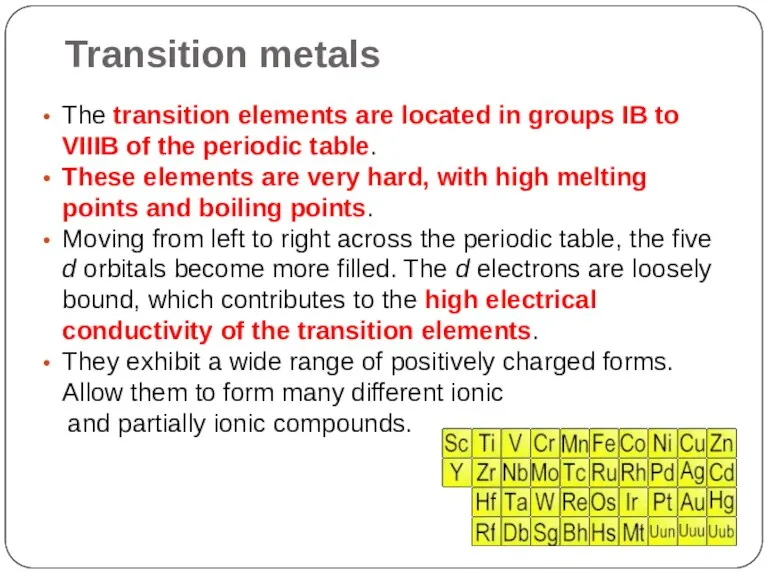
- 15. Transition metals The transition elements are located in groups IB to VIIIB of the periodic table.
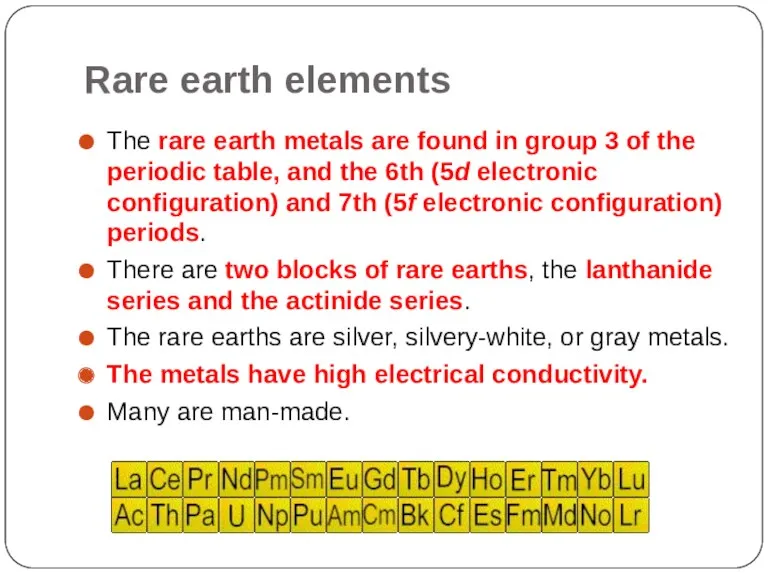
- 16. Rare earth elements The rare earth metals are found in group 3 of the periodic table,
- 17. Other than periods and groups, the table is divided into families
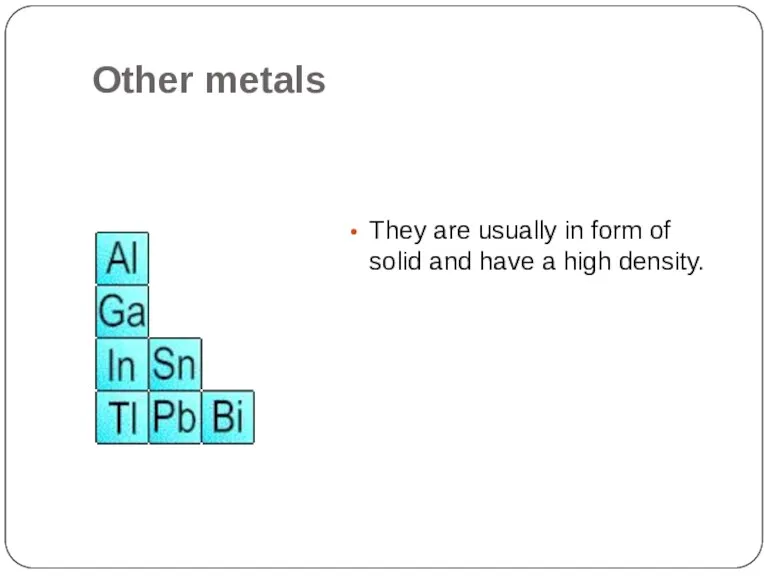
- 18. Other metals They are usually in form of solid and have a high density.
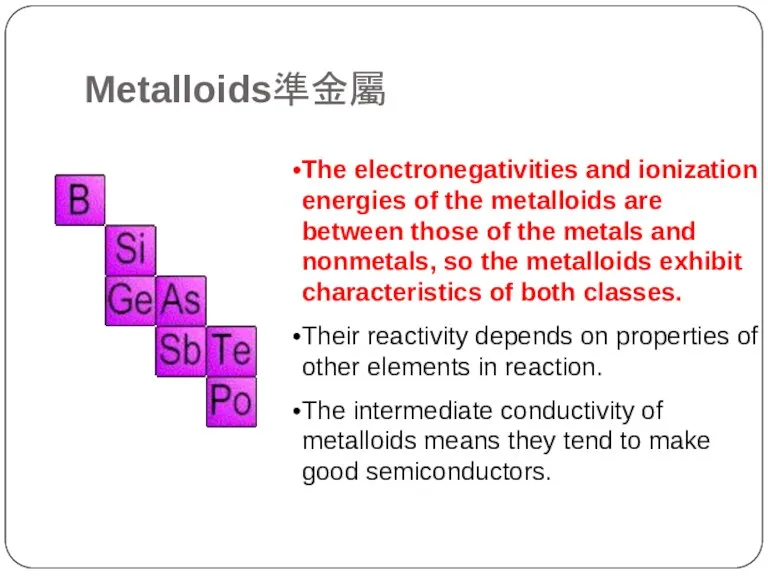
- 19. The electronegativities and ionization energies of the metalloids are between those of the metals and nonmetals,
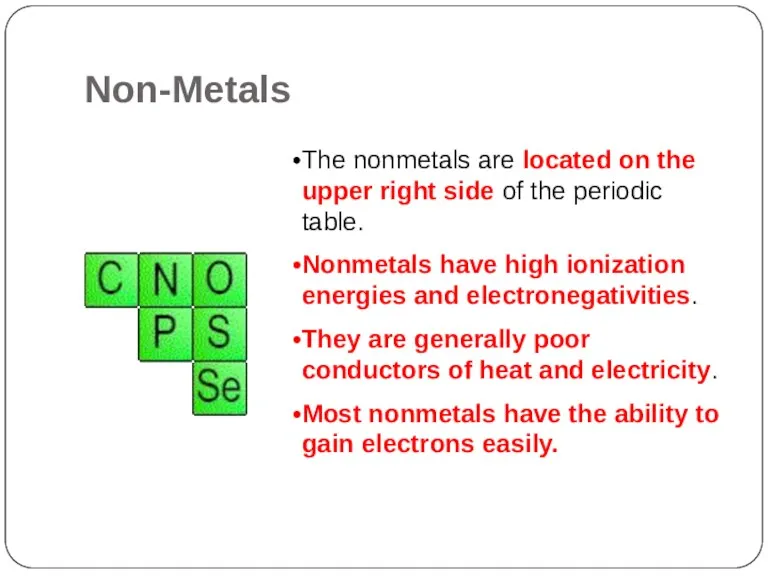
- 20. The nonmetals are located on the upper right side of the periodic table. Nonmetals have high
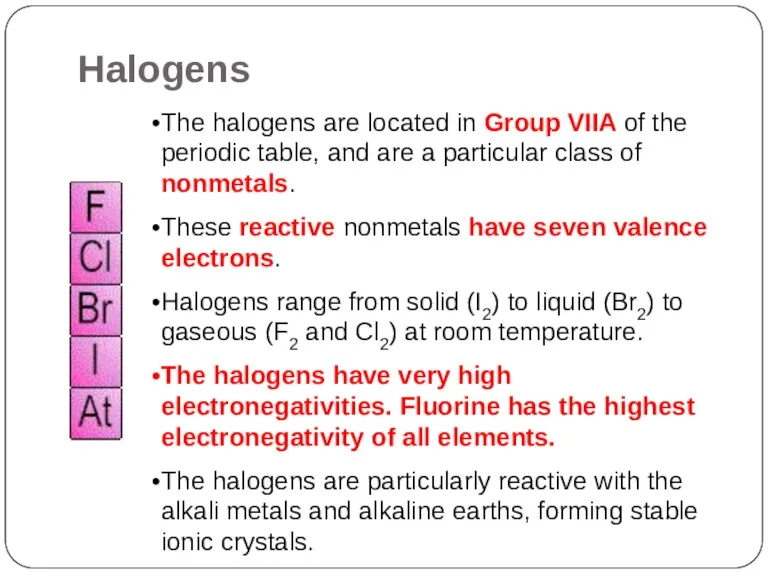
- 21. The halogens are located in Group VIIA of the periodic table, and are a particular class
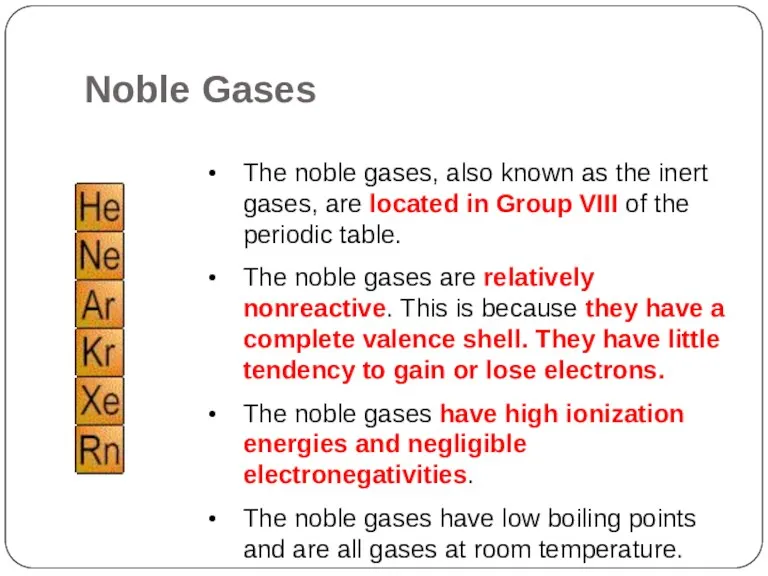
- 22. The noble gases, also known as the inert gases, are located in Group VIII of the
- 28. Скачать презентацию



 Синтетический каучук и его применение
Синтетический каучук и его применение Трансмиссионные масла
Трансмиссионные масла 20230816_belki_2
20230816_belki_2 Геохимия урана и тория в магматическом процессе
Геохимия урана и тория в магматическом процессе Вуглеводи
Вуглеводи Ртуть
Ртуть Единый государственный экзамен Химия 2021. Задание 3
Единый государственный экзамен Химия 2021. Задание 3 Химия нефти и газа
Химия нефти и газа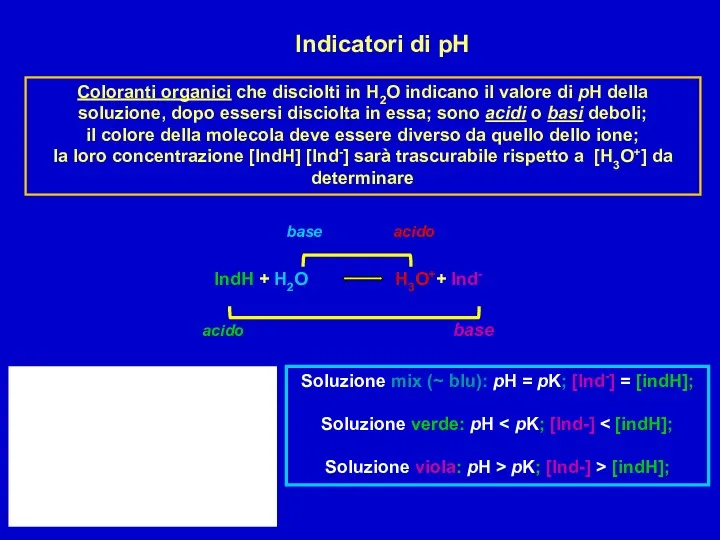 Indicatori di pH
Indicatori di pH Пластмаси. Класифікація пластмас
Пластмаси. Класифікація пластмас Серная кислота
Серная кислота Александр Евгеньевич Ферсман
Александр Евгеньевич Ферсман Метал конструкциялық материалдар
Метал конструкциялық материалдар Місце хімії серед наук про природу
Місце хімії серед наук про природу Оксид углерода II. Угарный газ
Оксид углерода II. Угарный газ Углерод, как химический элемент и простое вещество
Углерод, как химический элемент и простое вещество Получение Н2, О2, щелочей
Получение Н2, О2, щелочей Беттік активті заттардың беттік қасиеттері
Беттік активті заттардың беттік қасиеттері Химия элементов VIIA группы
Химия элементов VIIA группы Химия и проблемы охраны окружающей среды
Химия и проблемы охраны окружающей среды Классы органических соединений
Классы органических соединений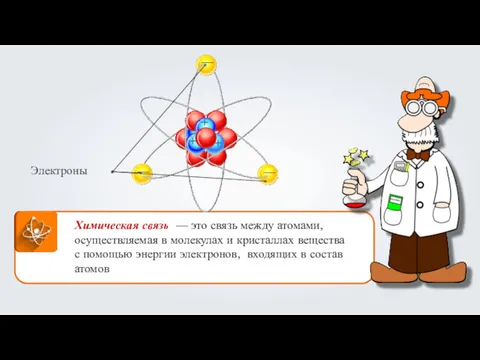 Взаимодействие атомов элементов-металлов и элементов-неметаллов между собой. Ионная связь. (Тема 10)
Взаимодействие атомов элементов-металлов и элементов-неметаллов между собой. Ионная связь. (Тема 10) Формы парфюмерно-косметической продукции
Формы парфюмерно-косметической продукции Химические процессы зоны гипергенеза
Химические процессы зоны гипергенеза Состав и классификация магматических горных пород
Состав и классификация магматических горных пород Активные формы кислорода. Антиоксиданты их физиологическая роль
Активные формы кислорода. Антиоксиданты их физиологическая роль Химия нефти и газа. Лекция № 1
Химия нефти и газа. Лекция № 1 Зависят ли свойства предельных одноатомных спиртов от их химического строения?
Зависят ли свойства предельных одноатомных спиртов от их химического строения?