Содержание
- 2. Li-ion Technology: where are we today Although tremendous progress has been made over the last couple
- 3. Sources of Thermal Instability The three main battery components (anode, cathode, electrolyte etc) all jointly contribute
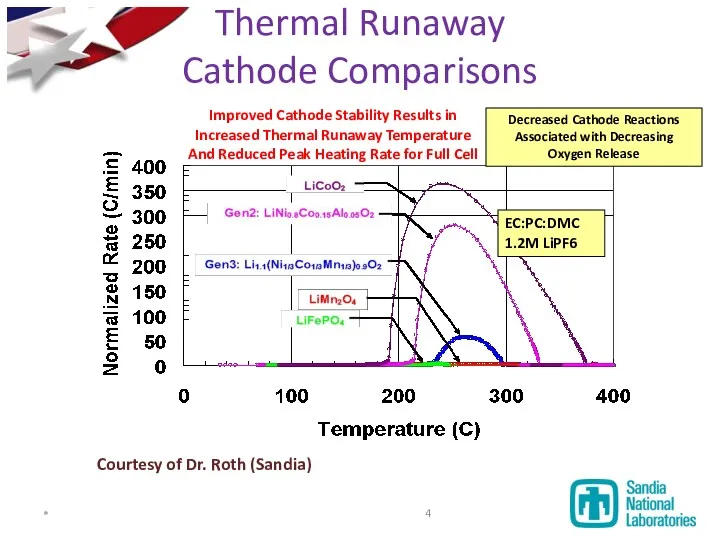
- 4. Improved Cathode Stability Results in Increased Thermal Runaway Temperature And Reduced Peak Heating Rate for Full
- 5. Potential path forward to overcoming the constraints Replacement of carbon materials with Nano-particulate metal, semi-metal, intermetallic
- 6. Sony successfully used metal composite anode, showed higher capacity Intermetallic compounds may hold the key for
- 7. Sony’s hybrid lithium-ion rechargeable battery *
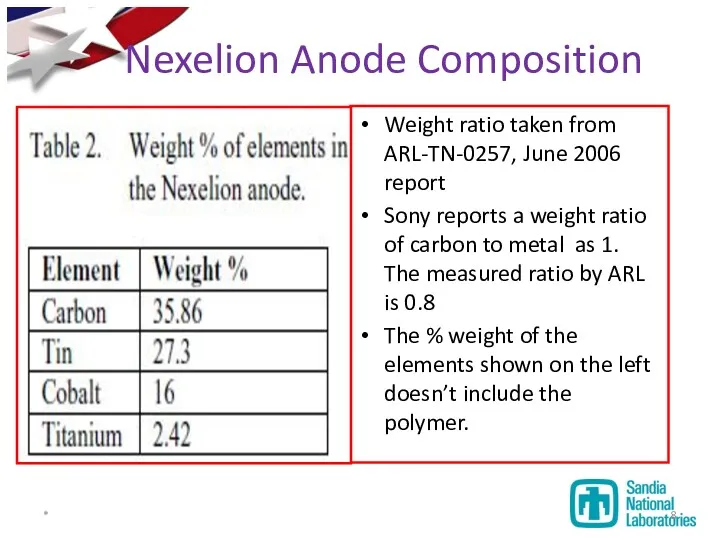
- 8. Weight ratio taken from ARL-TN-0257, June 2006 report Sony reports a weight ratio of carbon to
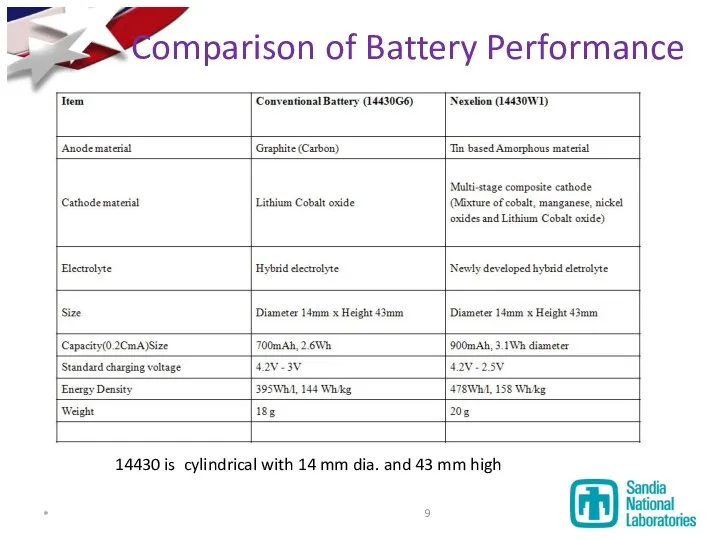
- 9. Comparison of Battery Performance 14430 is cylindrical with 14 mm dia. and 43 mm high *
- 10. Only 50% of the Li content can be taken out before the structure collapses Lower capacity
- 11. Ways to Improving Cathode Performance Increasing Energy Density Investigate high voltage cathodes that can deliver all
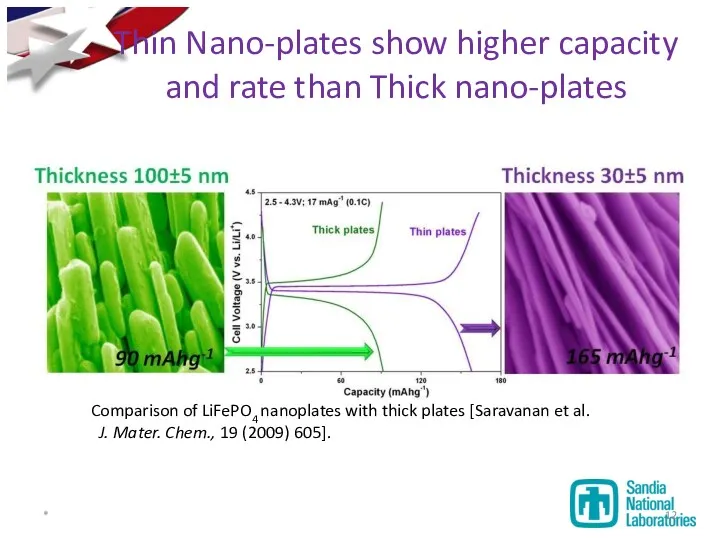
- 12. Thin Nano-plates show higher capacity and rate than Thick nano-plates *
- 13. AlF3 Coated Electrodes The surface coating of electrodes seem to improve capacity retention and performance over
- 14. Potential Cathode Materials Olivine based phosphates systems (LiMPO4 where M = Mn, Ni) that can deliver
- 15. Electrolyte (solvent + salt) The state-of-the-art electrolytes for Li-ion cells contain a blend of organic carbonate
- 16. New Solvents New fluoro solvents are being investigated as nonflammable solvents Solvent with a F to
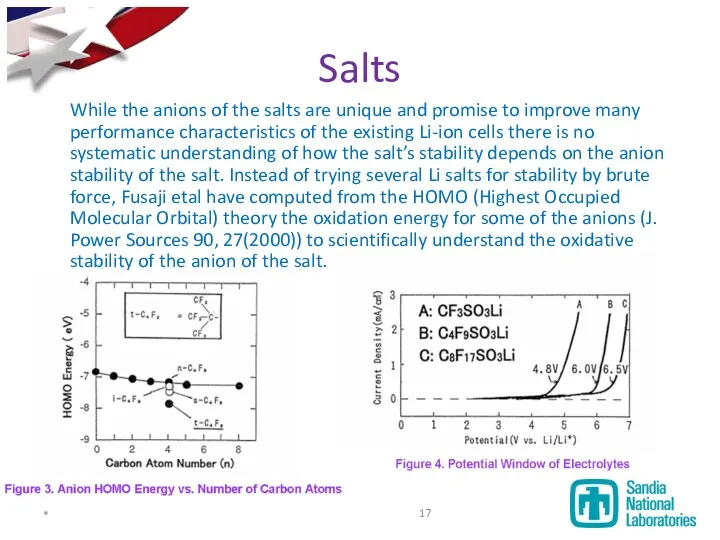
- 17. Salts While the anions of the salts are unique and promise to improve many performance characteristics
- 19. Скачать презентацию



 Качество Кубани
Качество Кубани Суть управления в обществе и его уровни
Суть управления в обществе и его уровни Пятна
Пятна Асептика и антисептика 222
Асептика и антисептика 222 Характеристика проблемного поля состояния социального предпринимательства в регионах Российской Федерации
Характеристика проблемного поля состояния социального предпринимательства в регионах Российской Федерации Презентация Метод детского экспериментирования
Презентация Метод детского экспериментирования Физкультминутка к уроку татарского языка Диск
Физкультминутка к уроку татарского языка Диск 20231203_cash_flow_denezhnyy_potok_1
20231203_cash_flow_denezhnyy_potok_1 Чувствительная функция нервной системы. Чувствительность и ее расстройства. Боль. Ноцицептивные и антиноцицептивные системы мозга
Чувствительная функция нервной системы. Чувствительность и ее расстройства. Боль. Ноцицептивные и антиноцицептивные системы мозга Классификация холодильных машин
Классификация холодильных машин Акционерное общество Всероссийский нефтегазовый научно-исследовательский институт имени академика А.П.Крылова
Акционерное общество Всероссийский нефтегазовый научно-исследовательский институт имени академика А.П.Крылова Балалардағы жүрек ырғағының бұзылысы
Балалардағы жүрек ырғағының бұзылысы Коэффициент корреляции τ Кендалла
Коэффициент корреляции τ Кендалла Миссия и цели организации
Миссия и цели организации Арбитраж. 9 поток
Арбитраж. 9 поток С годом российского депутата
С годом российского депутата Президентские гранты на развитие гражданского общества. Социальное проектирование
Президентские гранты на развитие гражданского общества. Социальное проектирование Компания LG Group
Компания LG Group Новые технологии дорожного строительства97-2003
Новые технологии дорожного строительства97-2003 Сельское хозяйство, его отраслевая структура
Сельское хозяйство, его отраслевая структура Корпоративное управление
Корпоративное управление Методические рекомендации по разработке и оформлению дополнительной общеобразовательной общеразвивающей программы
Методические рекомендации по разработке и оформлению дополнительной общеобразовательной общеразвивающей программы Взаимодействие с родителями при работе над проектом:Наш родной город -Колпино.
Взаимодействие с родителями при работе над проектом:Наш родной город -Колпино. Немного о своей работе
Немного о своей работе 5 игр на знакомство
5 игр на знакомство Работа в MS Visio 2016. Фигуры (часть 2)
Работа в MS Visio 2016. Фигуры (часть 2) Камера приема очистного устройства трубопроводов
Камера приема очистного устройства трубопроводов Жалобная книга природы
Жалобная книга природы