Содержание
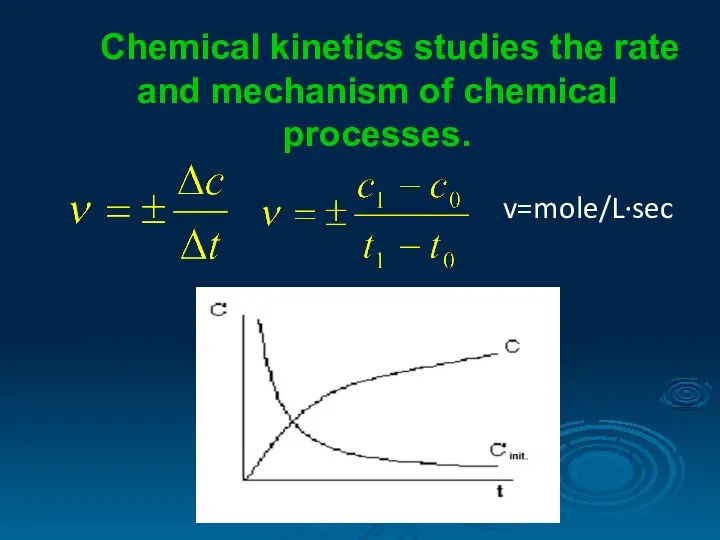
- 2. Chemical kinetics studies the rate and mechanism of chemical processes. v=mole/L∙sec
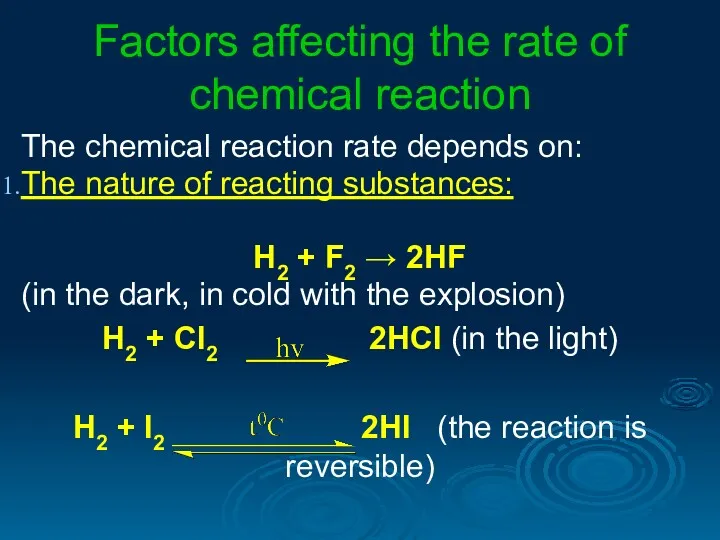
- 3. Factors affecting the rate of chemical reaction The chemical reaction rate depends on: The nature of
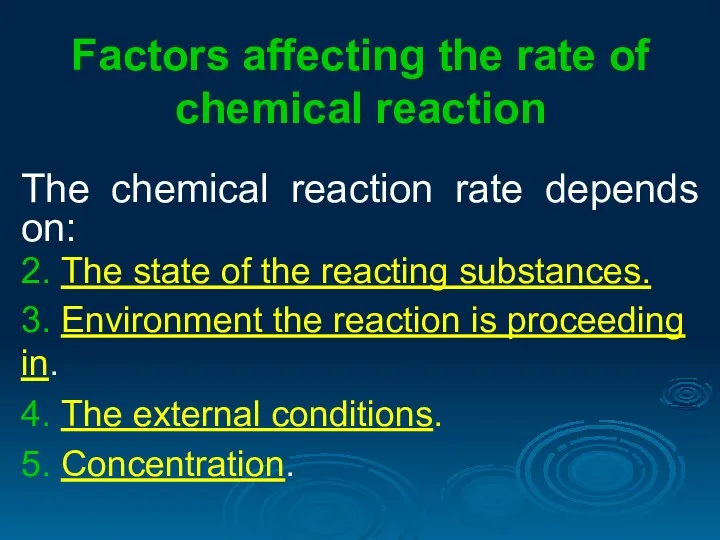
- 4. Factors affecting the rate of chemical reaction The chemical reaction rate depends on: 2. The state
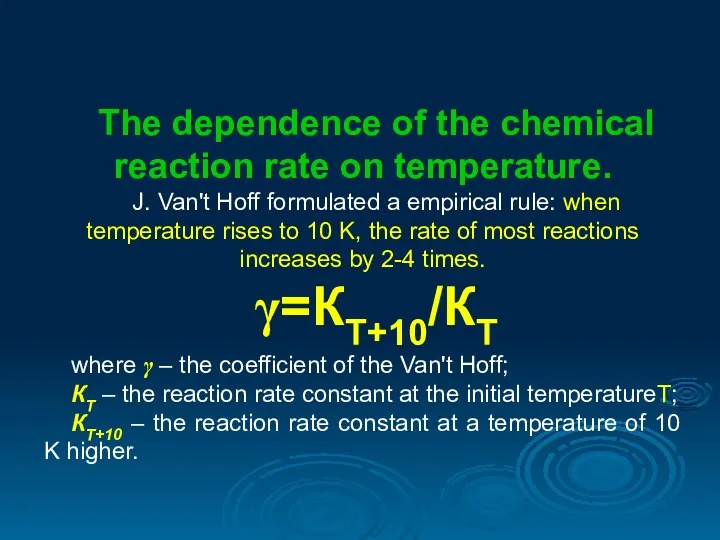
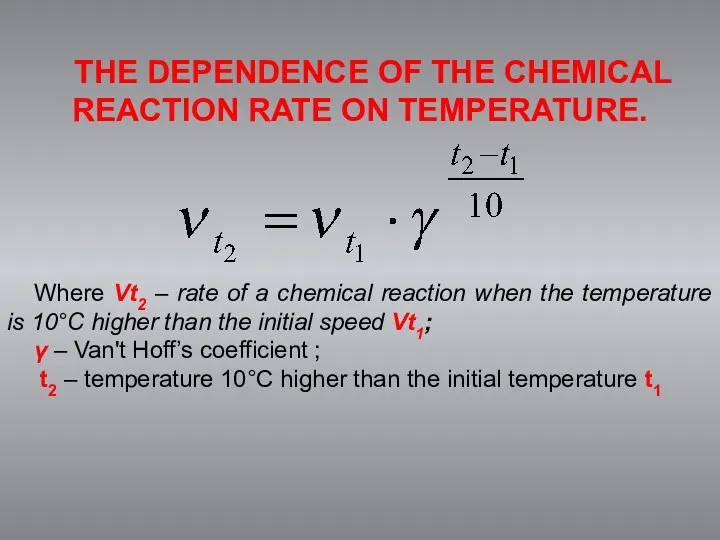
- 5. The dependence of the chemical reaction rate on temperature. J. Van't Hoff formulated a empirical rule:
- 6. THE DEPENDENCE OF THE CHEMICAL REACTION RATE ON TEMPERATURE. Where Vt2 – rate of a chemical
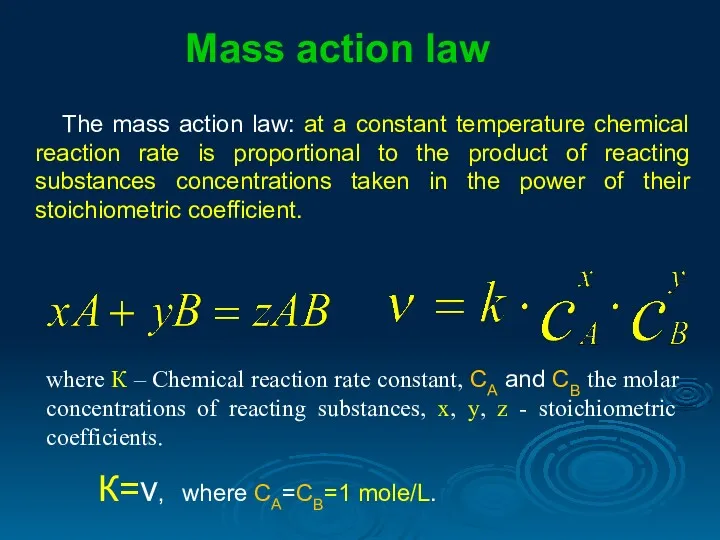
- 7. The mass action law: at a constant temperature chemical reaction rate is proportional to the product
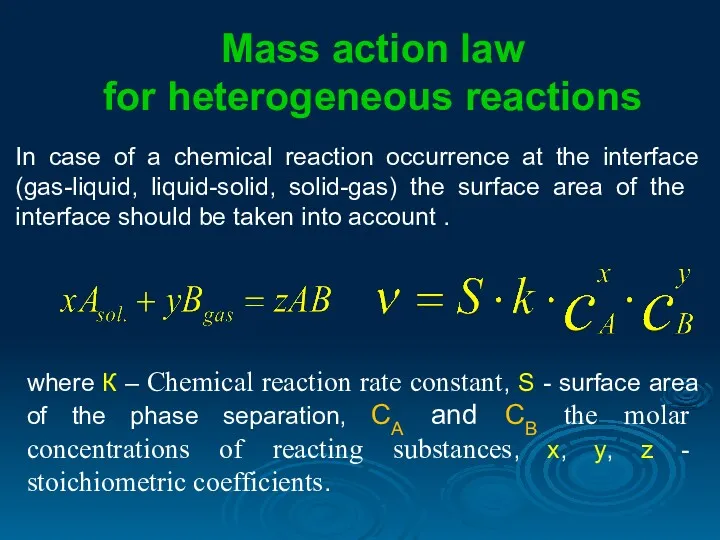
- 8. In case of a chemical reaction occurrence at the interface (gas-liquid, liquid-solid, solid-gas) the surface area
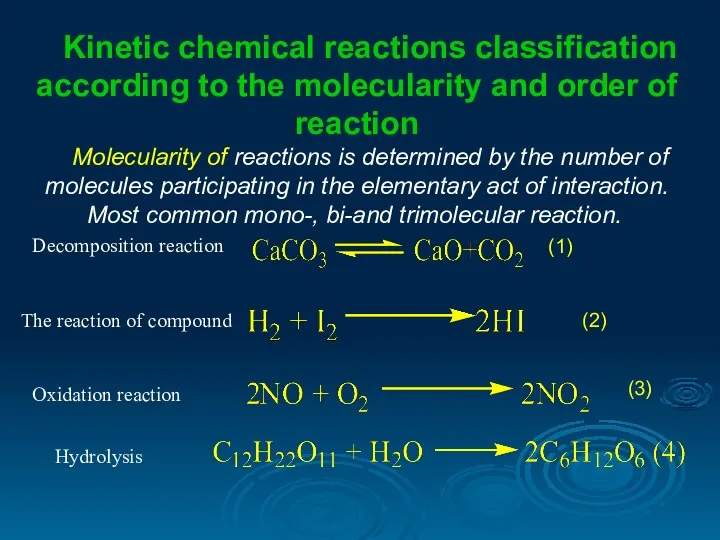
- 9. Kinetic chemical reactions classification according to the molecularity and order of reaction Molecularity of reactions is
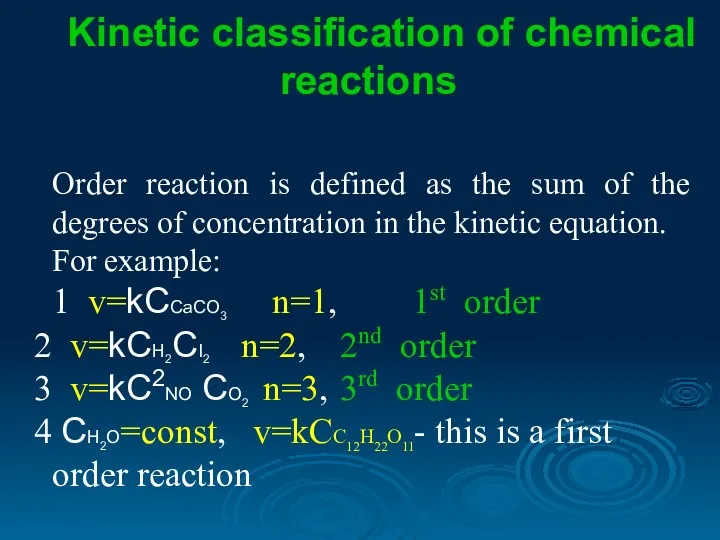
- 10. Kinetic classification of chemical reactions Order reaction is defined as the sum of the degrees of
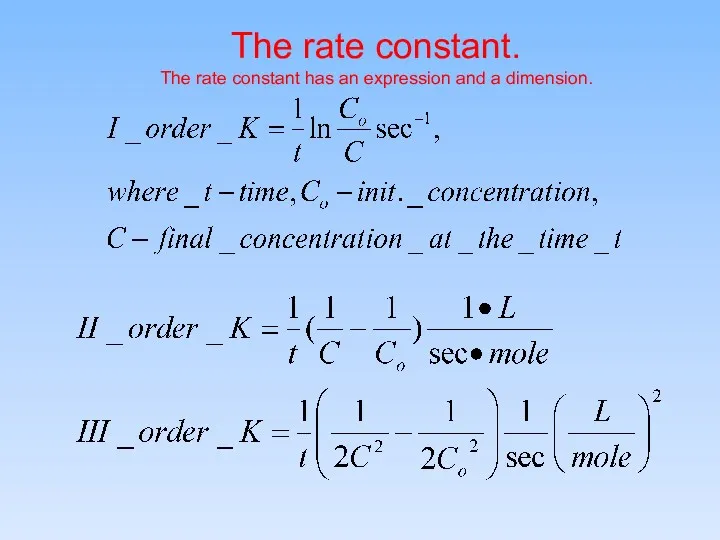
- 11. The rate constant. The rate constant has an expression and a dimension.
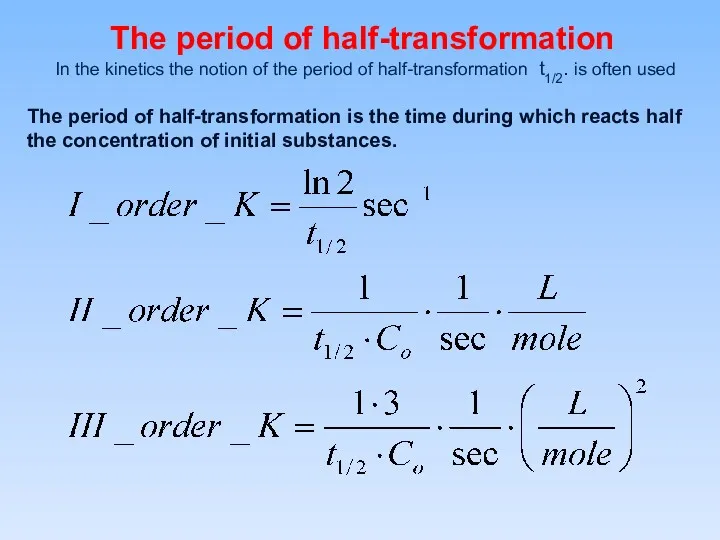
- 12. The period of half-transformation In the kinetics the notion of the period of half-transformation t1/2. is
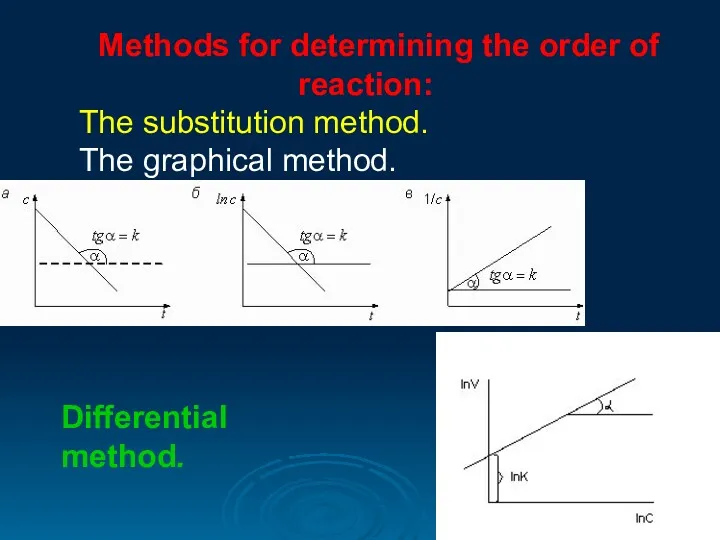
- 13. Methods for determining the order of reaction: The substitution method. The graphical method. Differential method.
- 14. The activation energy. A significant increase of the reaction rate as the temperature increases can be
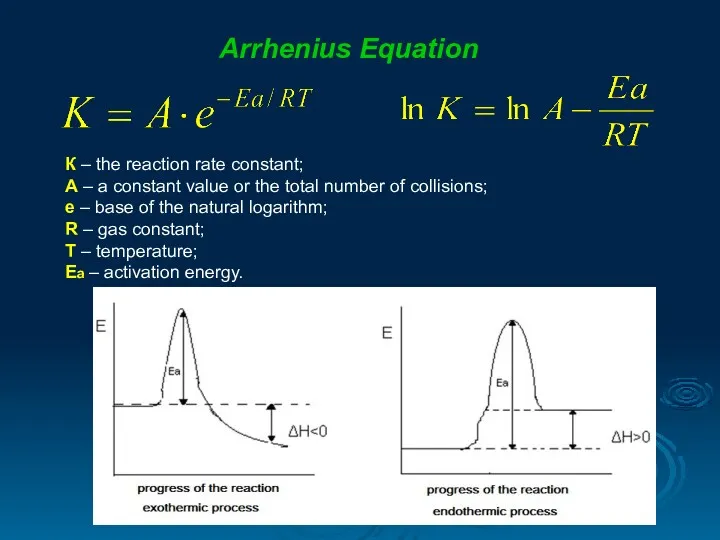
- 15. Arrhenius Equation К – the reaction rate constant; А – a constant value or the total
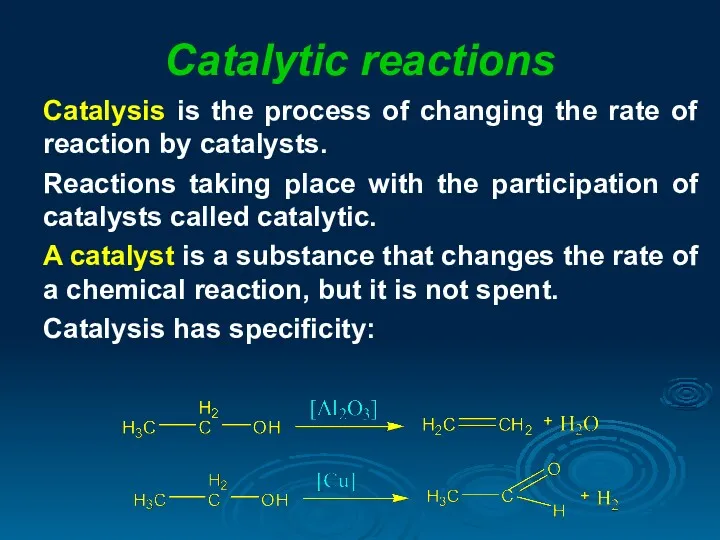
- 16. Catalytic reactions Catalysis is the process of changing the rate of reaction by catalysts. Reactions taking
- 17. Enzymes Enzymes are protein molecules able to accelerate the course of biochemical reactions. Other than enzymes-proteins
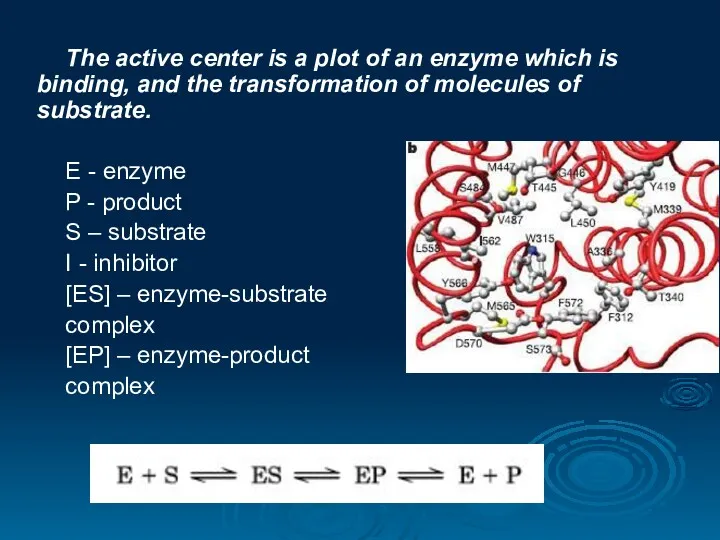
- 18. The active center is a plot of an enzyme which is binding, and the transformation of
- 19. Factors affecting the activity of the enzyme The concentration of the substrate. In 1913 Michaelis and
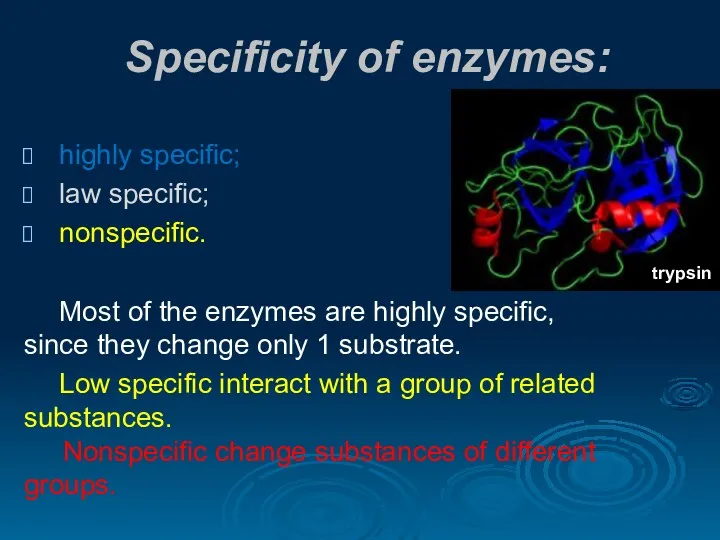
- 20. Specificity of enzymes: highly specific; law specific; nonspecific. Most of the enzymes are highly specific, since
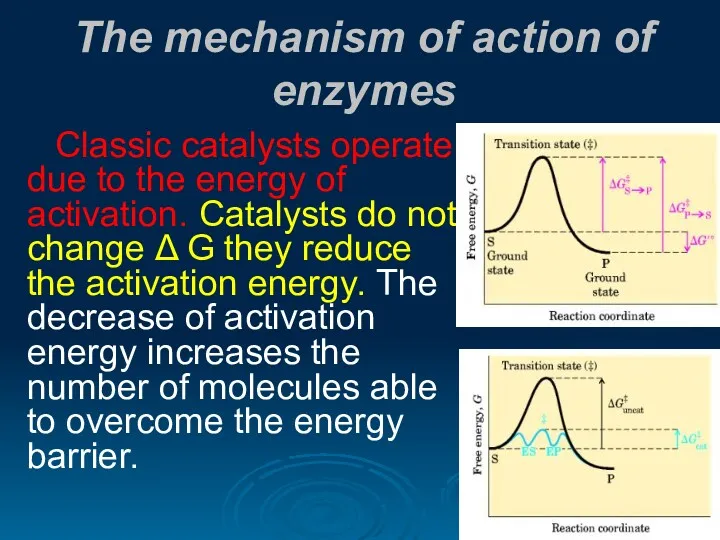
- 21. The mechanism of action of enzymes Classic catalysts operate due to the energy of activation. Catalysts
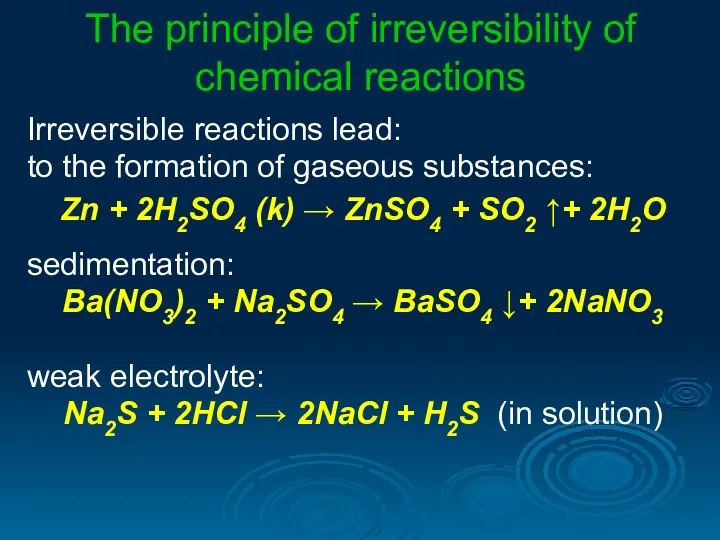
- 22. The principle of irreversibility of chemical reactions Irreversible reactions lead: to the formation of gaseous substances:
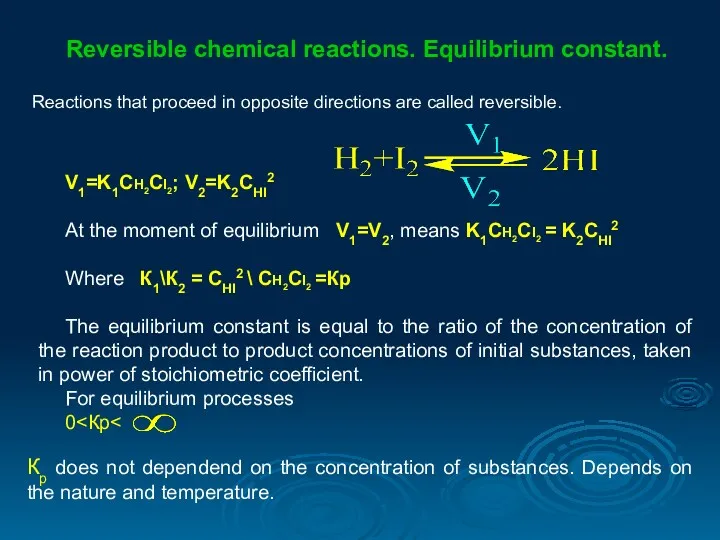
- 23. Reversible chemical reactions. Equilibrium constant. Reactions that proceed in opposite directions are called reversible. V1=K1CH2CI2; V2=K2CHI2
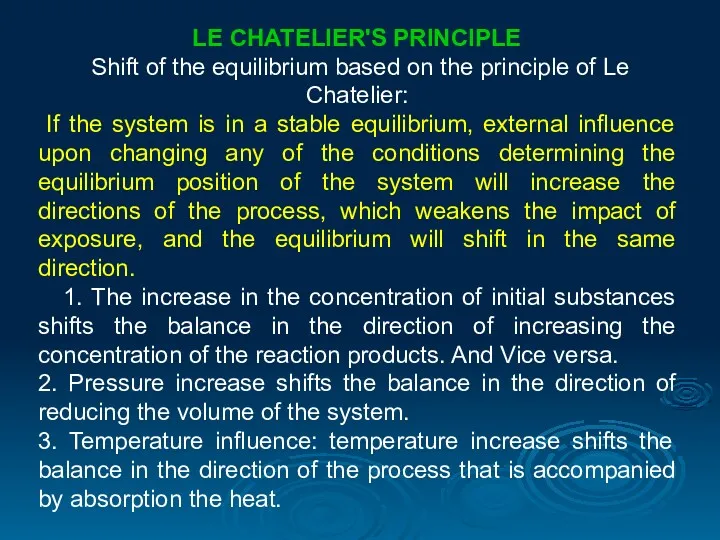
- 24. LE CHATELIER'S PRINCIPLE Shift of the equilibrium based on the principle of Le Chatelier: If the
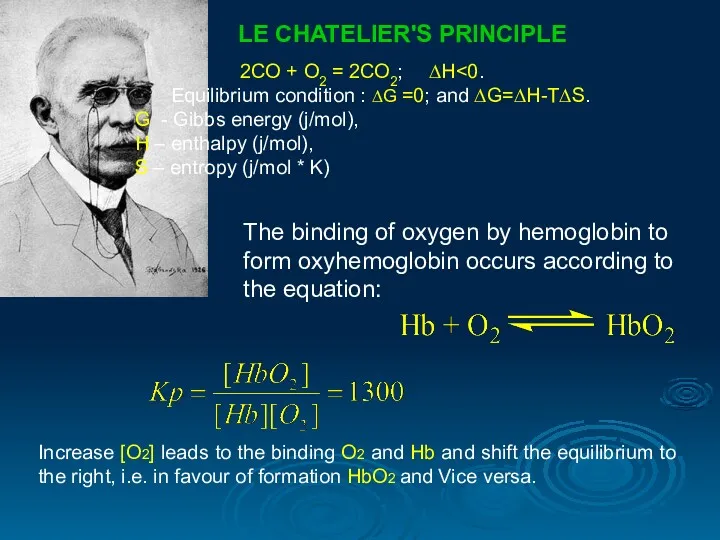
- 25. LE CHATELIER'S PRINCIPLE 2СО + О2 = 2СО2; ∆Н Equilibrium condition : ∆G =0; and ∆G=∆H-T∆S.
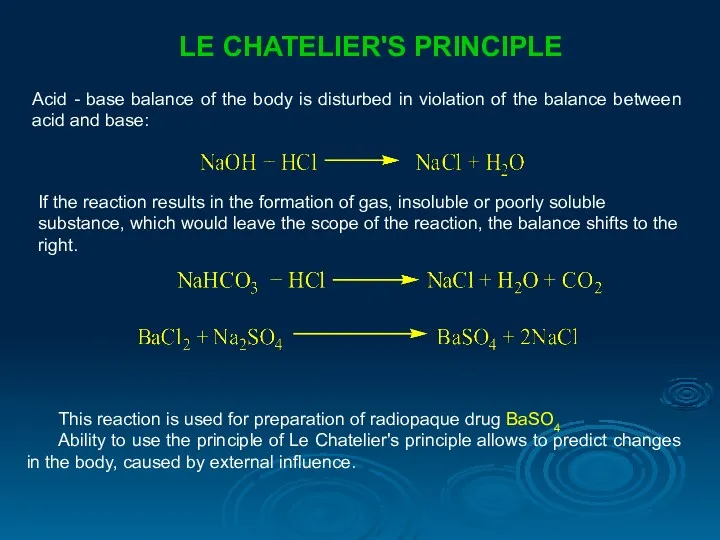
- 26. LE CHATELIER'S PRINCIPLE Acid - base balance of the body is disturbed in violation of the
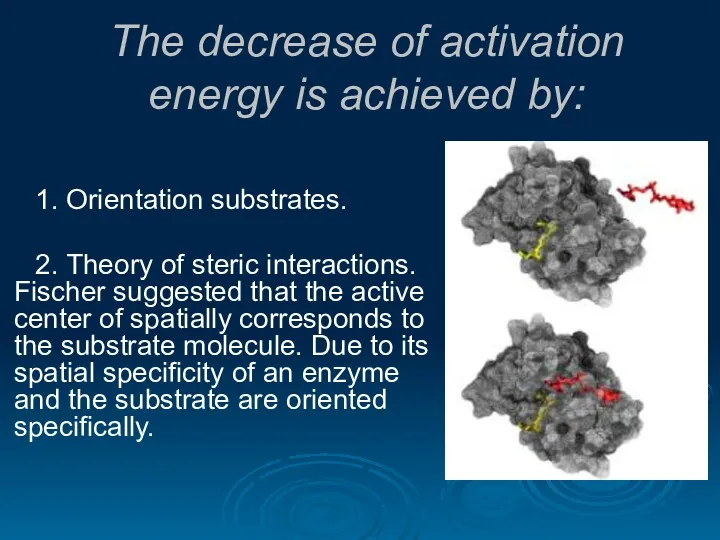
- 27. The decrease of activation energy is achieved by: 1. Orientation substrates. 2. Theory of steric interactions.
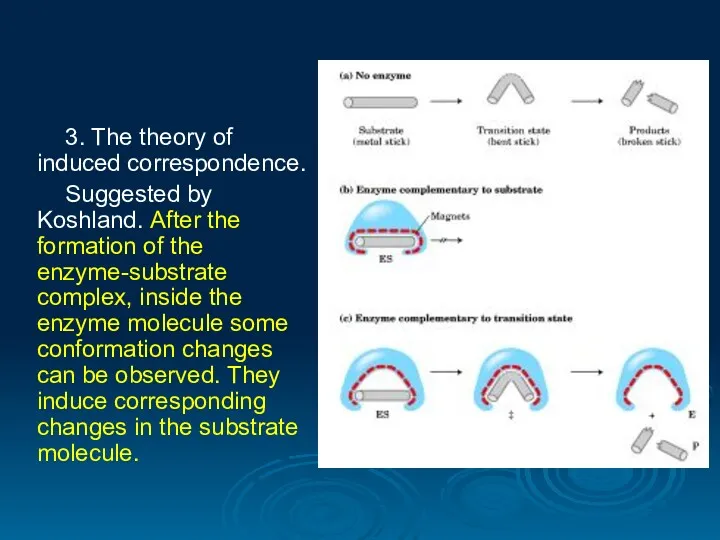
- 28. 3. The theory of induced correspondence. Suggested by Koshland. After the formation of the enzyme-substrate complex,
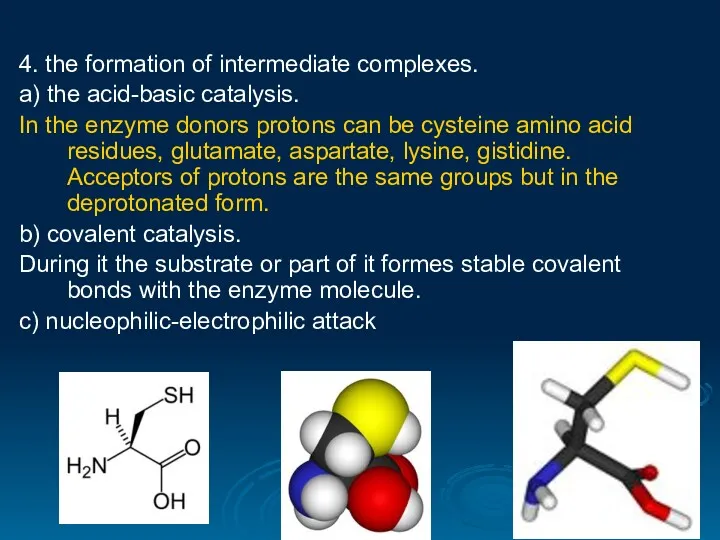
- 29. 4. the formation of intermediate complexes. а) the acid-basic catalysis. In the enzyme donors protons can
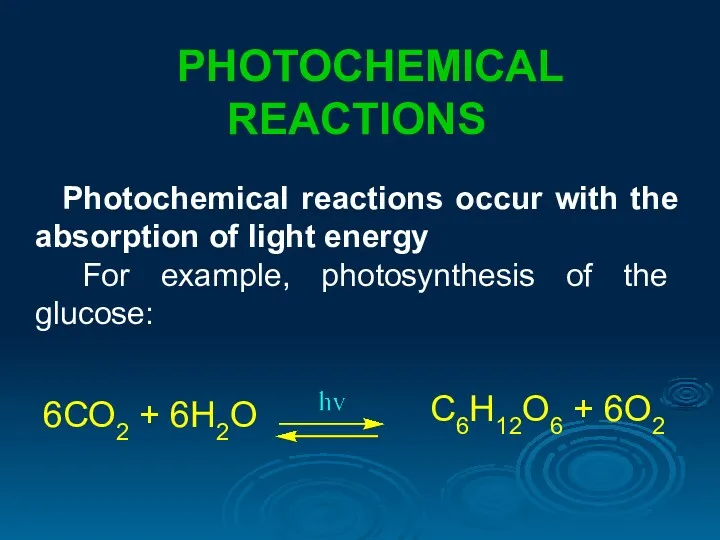
- 30. PHOTOCHEMICAL REACTIONS Photochemical reactions occur with the absorption of light energy For example, photosynthesis of the
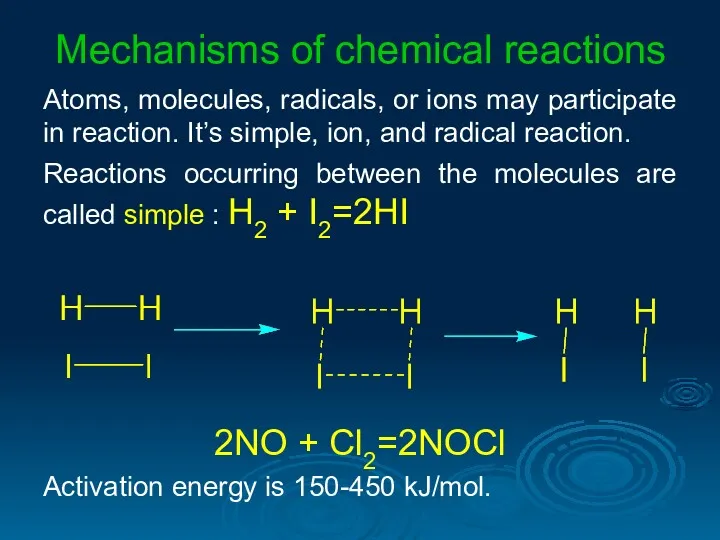
- 31. Mechanisms of chemical reactions Atoms, molecules, radicals, or ions may participate in reaction. It’s simple, ion,
- 33. Скачать презентацию


 Урок на тему:Аппликация светофор
Урок на тему:Аппликация светофор Робототехника. Системы автоматического управления устройств
Робототехника. Системы автоматического управления устройств Иван Яковлевич Билибин 4 августа 1876 г. – 7 февраля 1942 г
Иван Яковлевич Билибин 4 августа 1876 г. – 7 февраля 1942 г Медико-социальные аспекты работы с детьми школьного возраста
Медико-социальные аспекты работы с детьми школьного возраста Радиоприемные устройства. Регулировки
Радиоприемные устройства. Регулировки Электронный ЮУрГУ. Общая информация. Русский язык, как иностранный
Электронный ЮУрГУ. Общая информация. Русский язык, как иностранный Приведение параметров обмотки ротора к обмотке статора асинхронной машины. Векторная диаграмма асинхронного двигателя
Приведение параметров обмотки ротора к обмотке статора асинхронной машины. Векторная диаграмма асинхронного двигателя Ағаш қалдықтары үшін автономды қазандықтар
Ағаш қалдықтары үшін автономды қазандықтар Развитие речи детей старшего дошкольного возраста в сюжетно-ролевой игре
Развитие речи детей старшего дошкольного возраста в сюжетно-ролевой игре Участие граждан в политической жизни
Участие граждан в политической жизни Понятие уголовно-исполнительного права и его принципов, источники, нормы, правоотношения. Тема № 1
Понятие уголовно-исполнительного права и его принципов, источники, нормы, правоотношения. Тема № 1 Семья в современном обществе
Семья в современном обществе мастер-класс для учителей О чем говорит реклама на английском?
мастер-класс для учителей О чем говорит реклама на английском? Северо-западная Русь между востоком и западом
Северо-западная Русь между востоком и западом Роль увлечений в жизни ребенка
Роль увлечений в жизни ребенка презентация Викторина по географии для 8-9 классов
презентация Викторина по географии для 8-9 классов Командообразование
Командообразование Алгоритмизация и программирование
Алгоритмизация и программирование Презентация Автоматизация звука Р
Презентация Автоматизация звука Р Презентация Приобщение детей младшего возраста к русской народной культуре через театрализованную деятельность.
Презентация Приобщение детей младшего возраста к русской народной культуре через театрализованную деятельность. Статистика ЕГЭ. Результаты ЕГЭ по физике 2018 года и перспективы 2019 года
Статистика ЕГЭ. Результаты ЕГЭ по физике 2018 года и перспективы 2019 года Сочинение-рассуждение по фразе из текста. ОГЭ-9, 15-2
Сочинение-рассуждение по фразе из текста. ОГЭ-9, 15-2 Личность. Индивидуальность
Личность. Индивидуальность Профилактика вредных привычек
Профилактика вредных привычек Winemaking In Russia
Winemaking In Russia Системы охлаждения генераторов
Системы охлаждения генераторов Negative Externalities
Negative Externalities Милосердие, забота о слабых, взаимопомощь и отношение к ним разных религий
Милосердие, забота о слабых, взаимопомощь и отношение к ним разных религий