Содержание
- 2. CONTENT
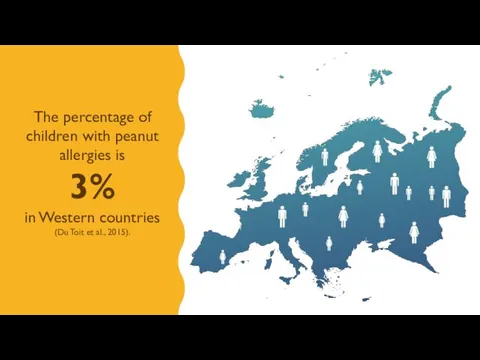
- 3. The percentage of children with peanut allergies is 3% in Western countries (Du Toit et al.,
- 4. PEANUT ALLERGY IS THE LEADING CAUSE OF ANAPHYLAXIS AND DEATH DUE TO FOOD ALLERGY (DU TOIT
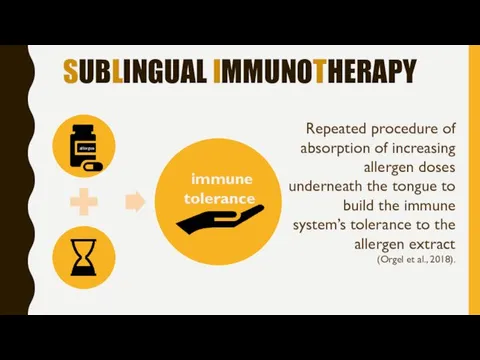
- 5. SUBLINGUAL IMMUNOTHERAPY Repeated procedure of absorption of increasing allergen doses underneath the tongue to build the
- 6. SUBLINGUAL DROPS consisted of peanut extract fully dissolved in 0.2% phenol and 50%-55% glycerinated saline (Kim
- 7. 1-YEAR SLIT OUTCOMES: Clinical desensitization Extended SLIT is required to assess : Higher level of clinical
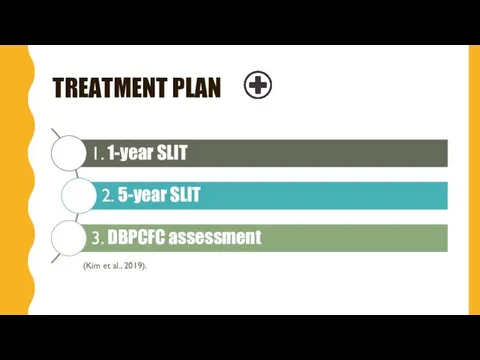
- 8. TREATMENT PLAN (Kim et al., 2019).
- 9. 1-YEAR SLIT 48 participants 6 months 6 months doses were biweekly increased from 0.25μg to 2000μg
- 10. EXTENDED SLIT (5 YEARS) 45 participants (3 withdrew) 5 years 2000μg daily maintenance dose of peanut
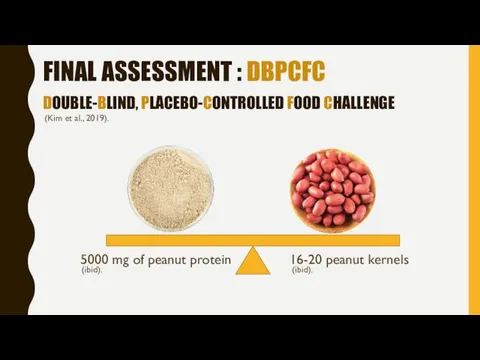
- 11. FINAL ASSESSMENT : DBPCFC DOUBLE-BLIND, PLACEBO-CONTROLLED FOOD CHALLENGE 5000 mg of peanut protein 16-20 peanut kernels
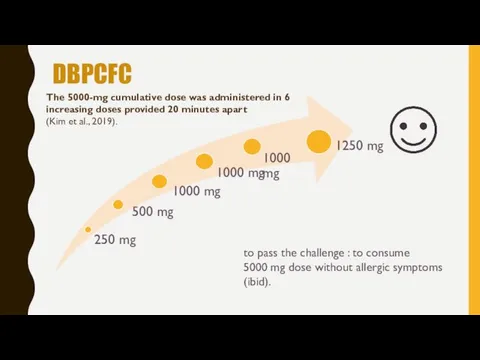
- 12. DBPCFC The 5000-mg cumulative dose was administered in 6 increasing doses provided 20 minutes apart (Kim
- 13. CUMULATIVE TOLERATED DOSE (MG) # OF SUBJECTS (37) (Kim et al., 2019, p. 4, FIG 2).
- 14. SUSTAINED UNRESPONSIVENESS (SU) (Kim et al., 2019).
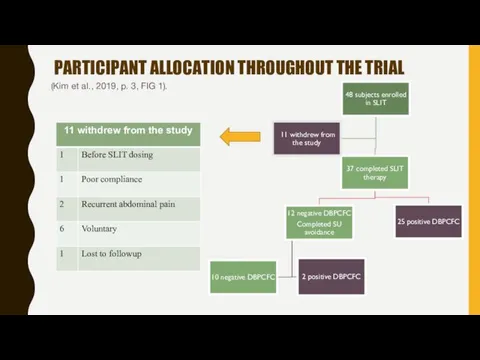
- 15. PARTICIPANT ALLOCATION THROUGHOUT THE TRIAL (Kim et al., 2019, p. 3, FIG 1).
- 16. SLIT SIDE EFFECTS (Kim et al., 2019 , p. 4, TABLE II).
- 17. EVALUATION effectiveness and safety of desensitization possible sustained unresponsiveness (SU) stability pattern of the post-SLIT desensitization
- 18. ANY QUESTIONS?
- 20. Скачать презентацию











 ПрезентацияМужские архетипы
ПрезентацияМужские архетипы презентация к уроку Основания 8 класс
презентация к уроку Основания 8 класс Разделы науки о языке
Разделы науки о языке Новое царство
Новое царство Растения в интерьере жилого дома
Растения в интерьере жилого дома Р. Сеф Весёлые стихи, 3класс
Р. Сеф Весёлые стихи, 3класс презентация группового занятия по патриотическому воспитанию
презентация группового занятия по патриотическому воспитанию Программа кормления животных ветеринарных врачей на территории дилера ИП Коробов
Программа кормления животных ветеринарных врачей на территории дилера ИП Коробов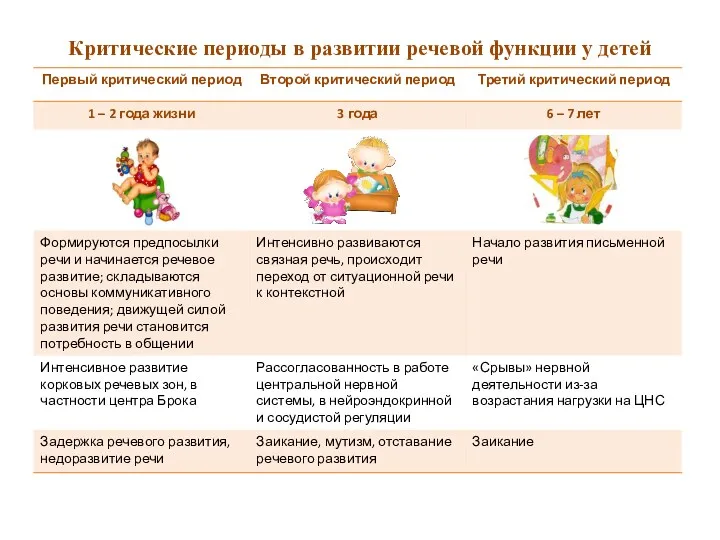 Информация для родителей. Критические периоды в развитии речевой функции у детей.
Информация для родителей. Критические периоды в развитии речевой функции у детей. Алгоритм сочинения загадок по опорным таблицам
Алгоритм сочинения загадок по опорным таблицам Презентация по химии для 9 класса по теме: Органические вещества.
Презентация по химии для 9 класса по теме: Органические вещества. Бойове застосування КЗА 86Ж6. Алгоритми обробки радiолокацiйної iнформацiї, виявлення та захоплення цiлей. (Тема 8.4)
Бойове застосування КЗА 86Ж6. Алгоритми обробки радiолокацiйної iнформацiї, виявлення та захоплення цiлей. (Тема 8.4) Презентация к статье Образовательное путешествие. Новые возможности
Презентация к статье Образовательное путешествие. Новые возможности Экологические проблемы Берингова моря
Экологические проблемы Берингова моря Портфолио педагога дополнительного образования (Презентация)
Портфолио педагога дополнительного образования (Презентация) Мастер-класс Народная кукла Кувадка
Мастер-класс Народная кукла Кувадка Сумматоры. Виды
Сумматоры. Виды DSM Food Specialties
DSM Food Specialties Wodospady iguazú - park narodowy Аrgentyny i Вrazylii
Wodospady iguazú - park narodowy Аrgentyny i Вrazylii 20230916_videoprezentatsiya_k_obobshchayushchemu_uroku_po_prirodovedeniyu_dlya_6_kl
20230916_videoprezentatsiya_k_obobshchayushchemu_uroku_po_prirodovedeniyu_dlya_6_kl Презентация. Климат Австралии, 7 класс.
Презентация. Климат Австралии, 7 класс. Устный счёт
Устный счёт Мастер - класс по фелтингу Осенние листья
Мастер - класс по фелтингу Осенние листья Мы - граждане России
Мы - граждане России Презентация Весёлый гномик
Презентация Весёлый гномик Устройство и эксплуатация солнечных батарей
Устройство и эксплуатация солнечных батарей Поэтапное рисование кошки
Поэтапное рисование кошки Кузнецкая крепость Диск
Кузнецкая крепость Диск