Содержание
- 2. General Properties of 2A *They give up electrons easily. *They have +2 charge *They are not
- 3. OCCURRENCE Since the group 2A elements are relatively active metals, they occur in compounds in nature.
- 4. Calcium, Ca Calcium compounds are widely distributed in nature, occurring as limestone or marble (CaCO3), gypsum
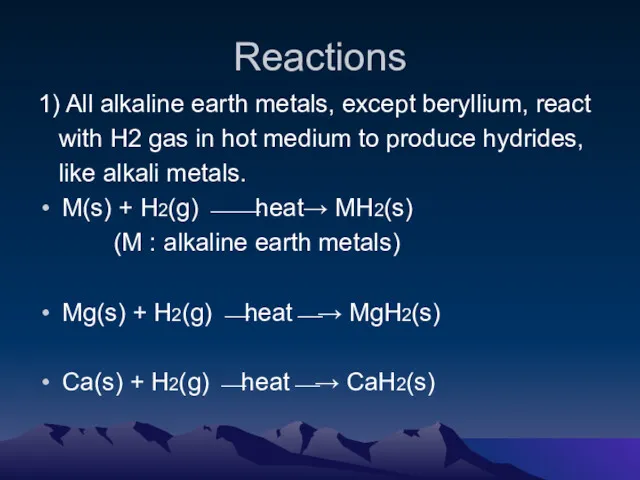
- 5. Reactions 1) All alkaline earth metals, except beryllium, react with H2 gas in hot medium to
- 6. 2) Ca, Sr and Ba react with water, like alkali metals, at room temperature to produce
- 7. 3. They form oxides as a result of their reactions with oxygen, in MO formula 2M(s)
- 8. 4. All alkaline earth metals give direct reactions with halogens to produce metal halides. M(s) +
- 9. 5. The reactions of the group 2A elements with acids like HCl and H2SO4, produce salts
- 11. Скачать презентацию







 Планетарная модель строения атома
Планетарная модель строения атома Количество вещества. Молярная масса. Молярный объем
Количество вещества. Молярная масса. Молярный объем Ансамбль пор в реальном твердом теле
Ансамбль пор в реальном твердом теле Чистые вещества и смеси
Чистые вещества и смеси Химический элемент фосфор
Химический элемент фосфор Переходные элементы
Переходные элементы Гетерофазный катализ. (Лекция 20)
Гетерофазный катализ. (Лекция 20) Живая» и «мёртвая» вода
Живая» и «мёртвая» вода Производство метанола. Физико-химические основы синтеза метанола. Современные катализаторы
Производство метанола. Физико-химические основы синтеза метанола. Современные катализаторы Реакции щелочно-земельных металлов (Группа 2) – Mg, Ca
Реакции щелочно-земельных металлов (Группа 2) – Mg, Ca Химические реакторы. Лекция №6
Химические реакторы. Лекция №6 Полисахариды: крахмал и целлюлоза
Полисахариды: крахмал и целлюлоза Лекция № 7. Конкурентные реакции у насыщенного атома
Лекция № 7. Конкурентные реакции у насыщенного атома Удобрения
Удобрения Соединения серы
Соединения серы Корунд. Разновидности корунда
Корунд. Разновидности корунда Электролитическая диссоциация. 9 класс
Электролитическая диссоциация. 9 класс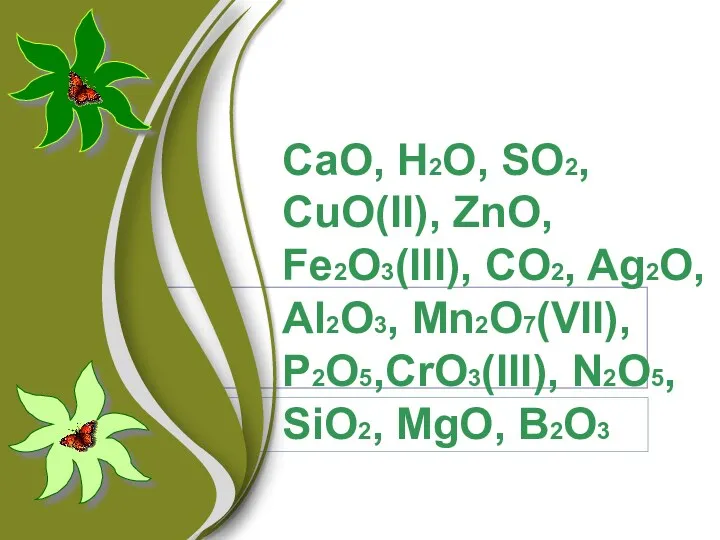 Оксиды. Классификация. Номенклатура. Свойства оксидов. Получение. Применение
Оксиды. Классификация. Номенклатура. Свойства оксидов. Получение. Применение Классификация и номенклатура неорганических веществ
Классификация и номенклатура неорганических веществ Основные классы неорганических веществ
Основные классы неорганических веществ Стратегия химической промышленности
Стратегия химической промышленности Золото. Виды золота
Золото. Виды золота Альдегиды и карбоновые кислоты
Альдегиды и карбоновые кислоты Основные классы неорганических веществ. Соединения химических элементов
Основные классы неорганических веществ. Соединения химических элементов Минералы. Свойства минералов
Минералы. Свойства минералов Серная кислота и ее соли
Серная кислота и ее соли Оксиды. 9 класс
Оксиды. 9 класс Кислые породы умеренно-щелочного ряда
Кислые породы умеренно-щелочного ряда