Содержание
- 2. EDUCATIONAL GOALS 1) Compare and contrast: mixtures and pure substances. solutions, suspensions, and colloids. 2) Understand,
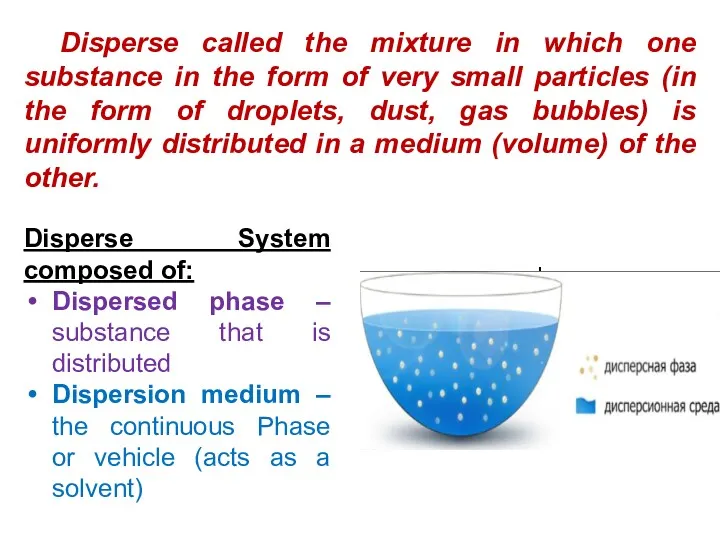
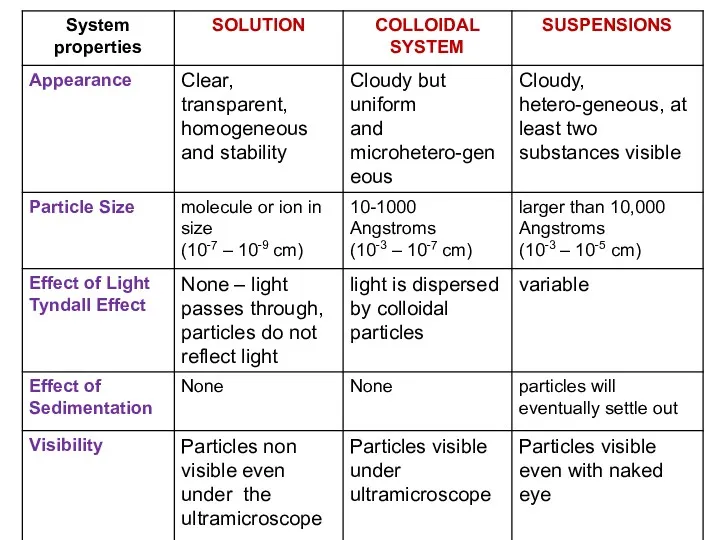
- 3. Disperse called the mixture in which one substance in the form of very small particles (in
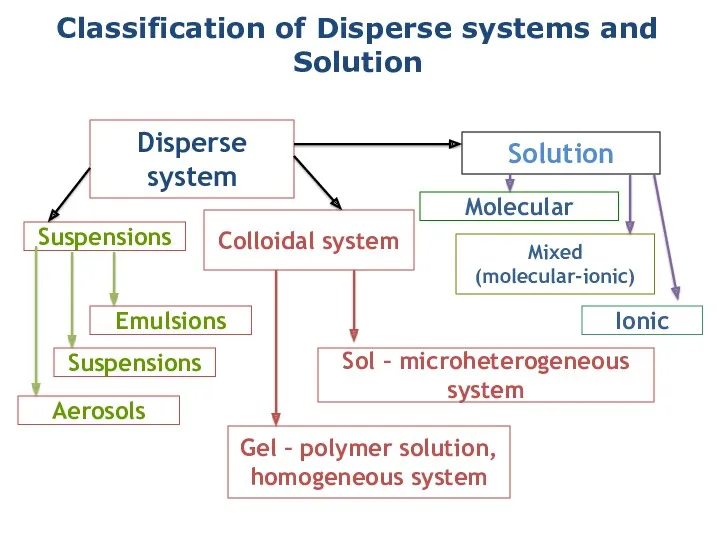
- 4. Classification of Disperse systems and Solution Disperse system Solution Suspensions Colloidal system Emulsions Suspensions Aerosols Gel
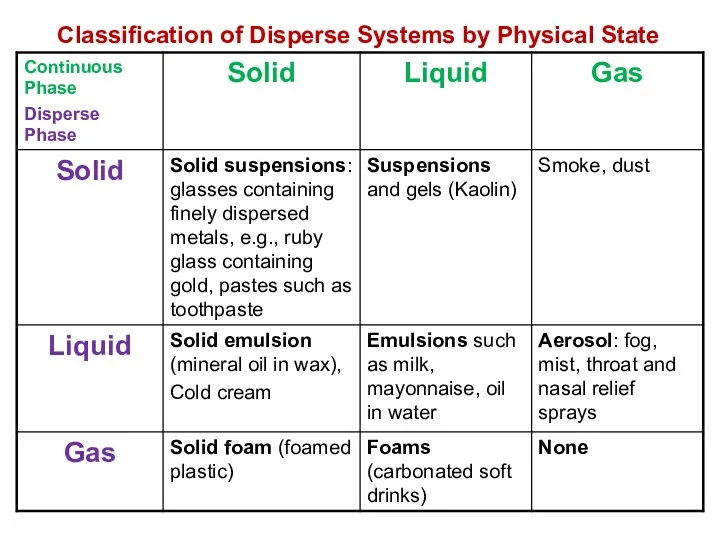
- 5. Classification of Disperse Systems by Physical State
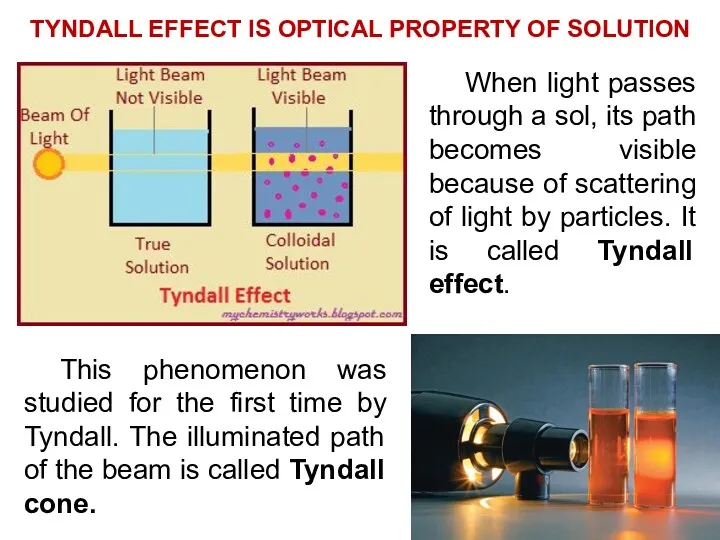
- 6. TYNDALL EFFECT IS OPTICAL PROPERTY OF SOLUTION This phenomenon was studied for the first time by
- 7. TYPES OF DISPERES SYSTEMS BY PARTICLE SIZE TRUE SOLUTION D COLLOIDAL SYSTEM D = 10-7 –
- 9. QUIZ ME NEXT 1 What is it a real solution? a pure substances in water compound
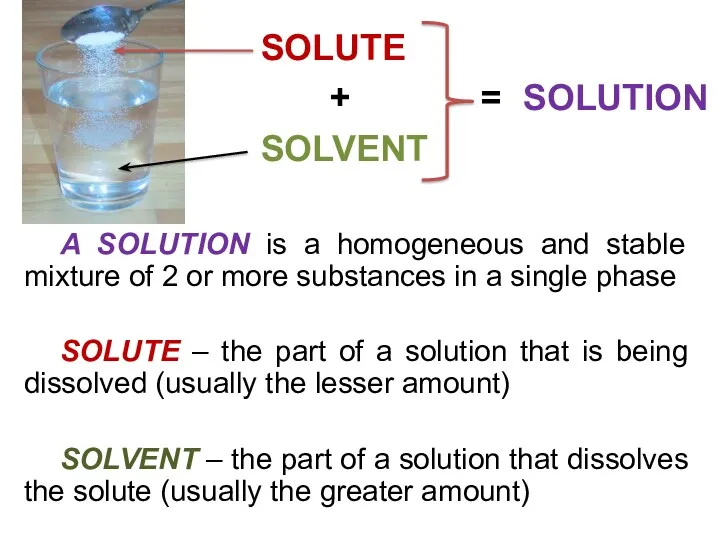
- 10. A SOLUTION is a homogeneous and stable mixture of 2 or more substances in a single
- 11. QUIZ ME NEXT 2 A solution consists of two parts. One part is the substance that
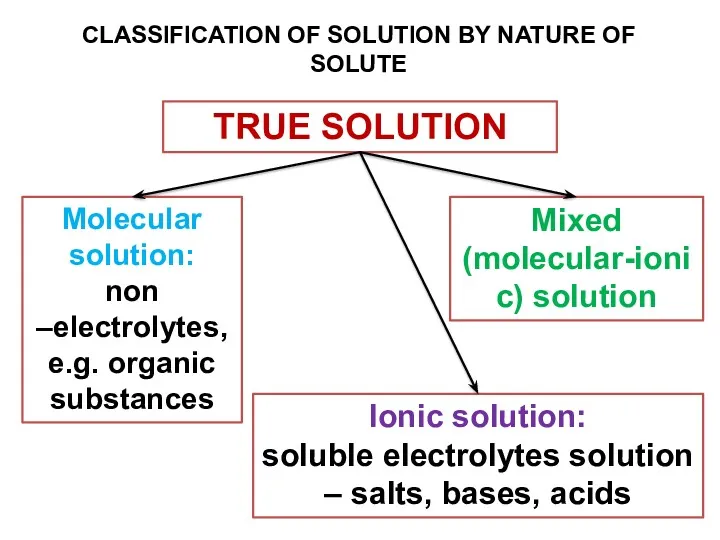
- 12. TRUE SOLUTION CLASSIFICATION OF SOLUTION BY NATURE OF SOLUTE Molecular solution: non –electrolytes, e.g. organic substances
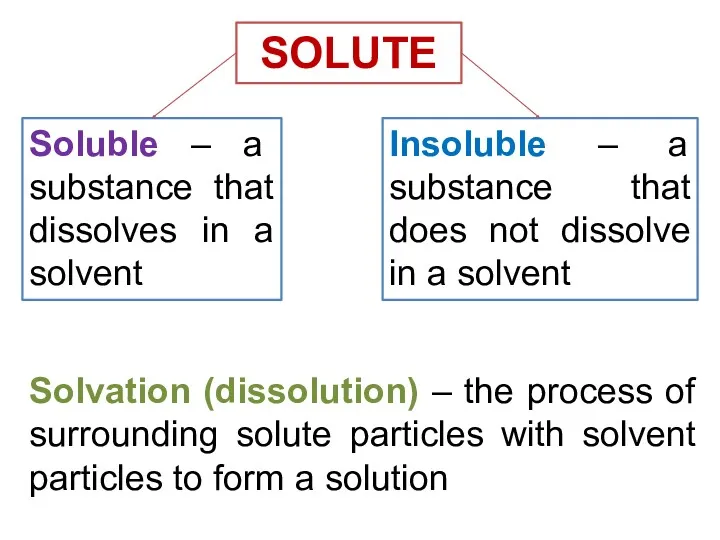
- 13. Solvation (dissolution) – the process of surrounding solute particles with solvent particles to form a solution
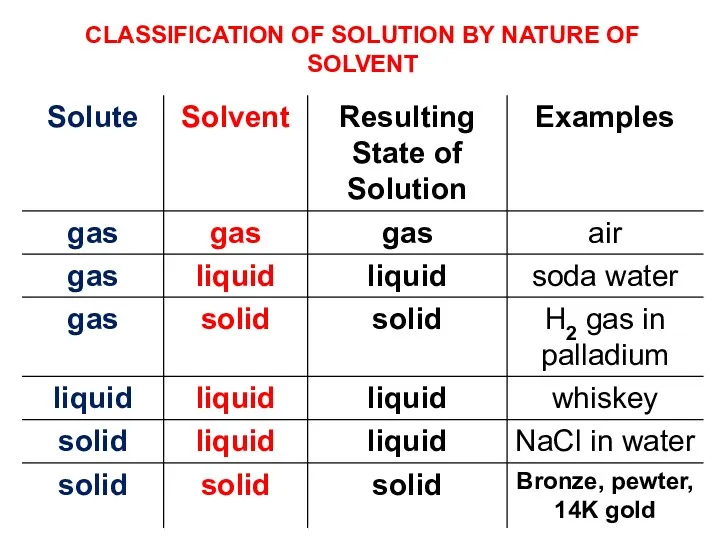
- 15. CLASSIFICATION OF SOLUTION BY NATURE OF SOLVENT
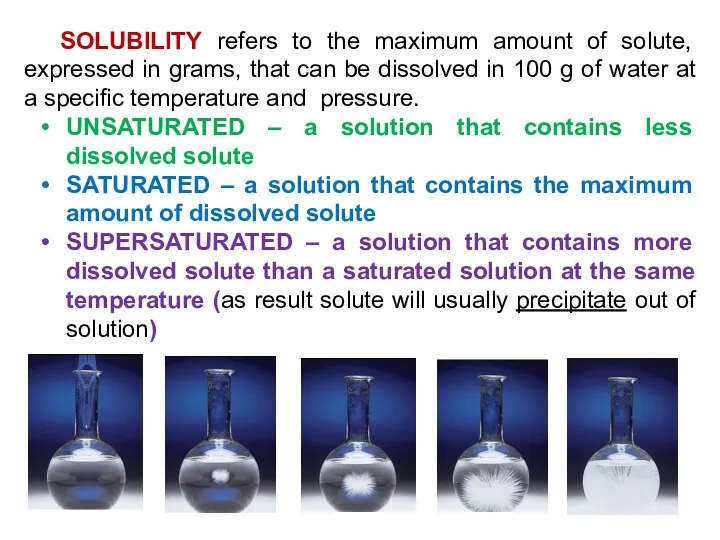
- 16. SOLUBILITY refers to the maximum amount of solute, expressed in grams, that can be dissolved in
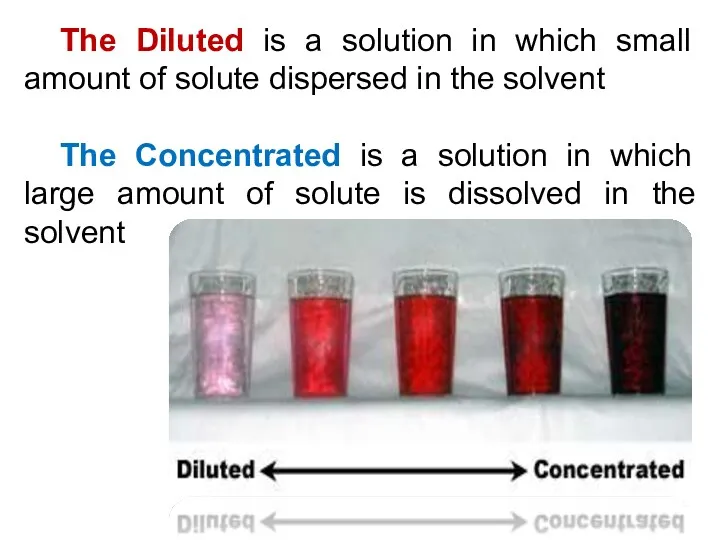
- 17. The Diluted is a solution in which small amount of solute dispersed in the solvent The
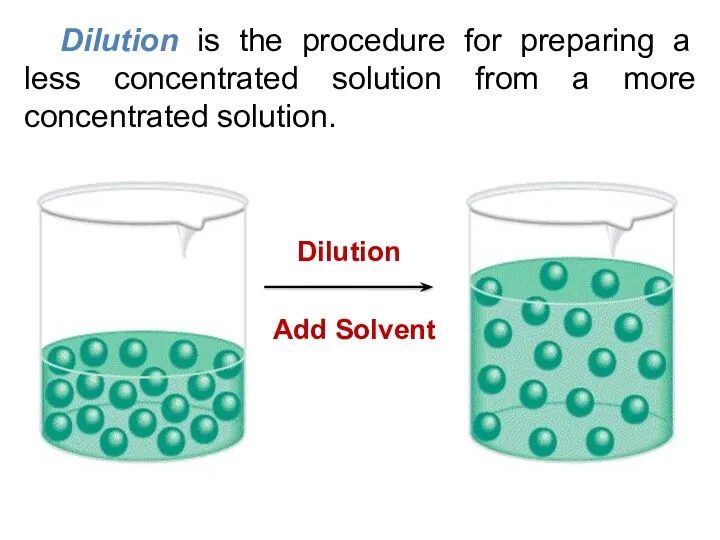
- 18. Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution.
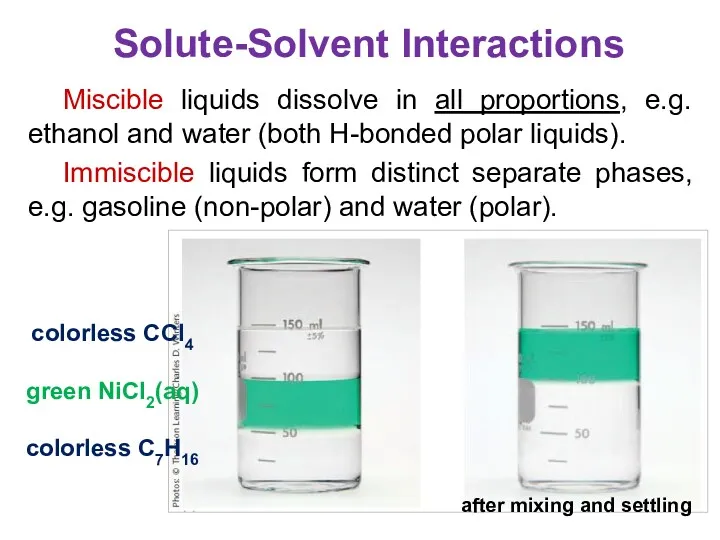
- 19. Miscible liquids dissolve in all proportions, e.g. ethanol and water (both H-bonded polar liquids). Immiscible liquids
- 20. Factors affecting solubility The nature of the solute and solvent: Polar substances tend to dissolve in
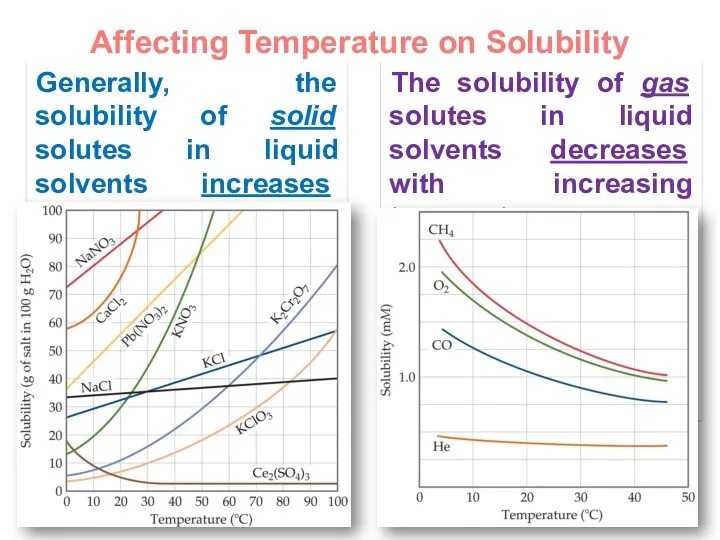
- 21. Affecting Temperature on Solubility Generally, the solubility of solid solutes in liquid solvents increases with increasing
- 22. Gases in Solution Increasing pressure above solution forces more gas to dissolve. The solubility of liquids
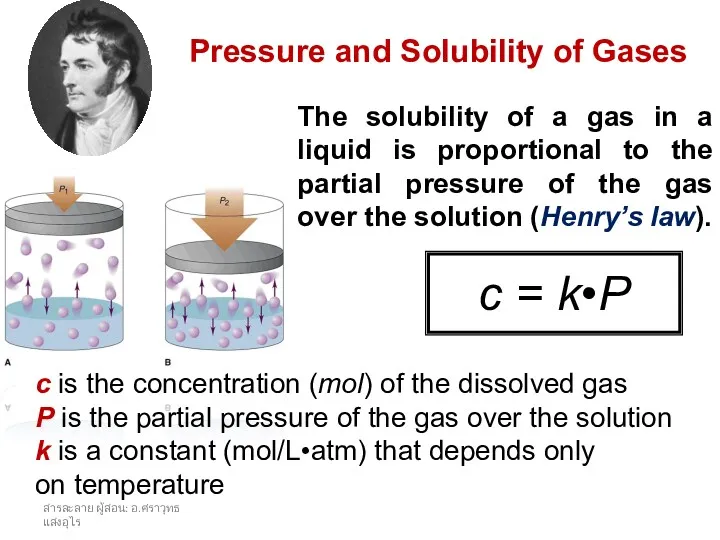
- 23. สารละลาย ผู้สอน: อ.ศราวุทธ แสงอุไร Pressure and Solubility of Gases The solubility of a gas in a
- 24. The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an
- 25. QUIZ ME NEXT 3 The amount of a solute dissolved in a given amount of solvent
- 26. The concentration of a solution is the amount of solute present in a given quantity of
- 27. 1) Percent composition by mass is the mass of the solute divided by the mass of
- 28. 3) Normality is equal to the gram equivalent weight of a solute per 1 liter of
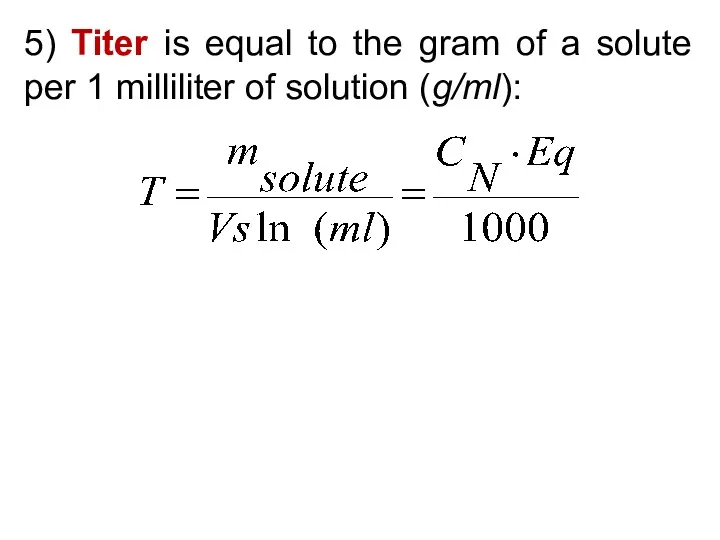
- 29. 5) Titer is equal to the gram of a solute per 1 milliliter of solution (g/ml):
- 31. Скачать презентацию








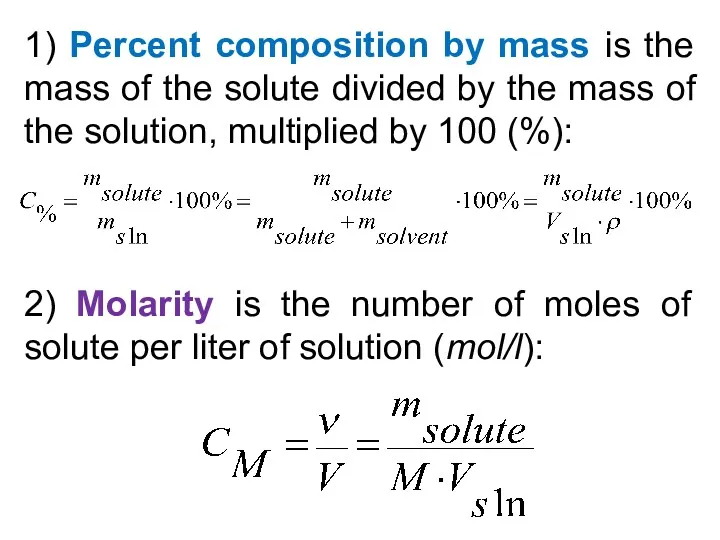
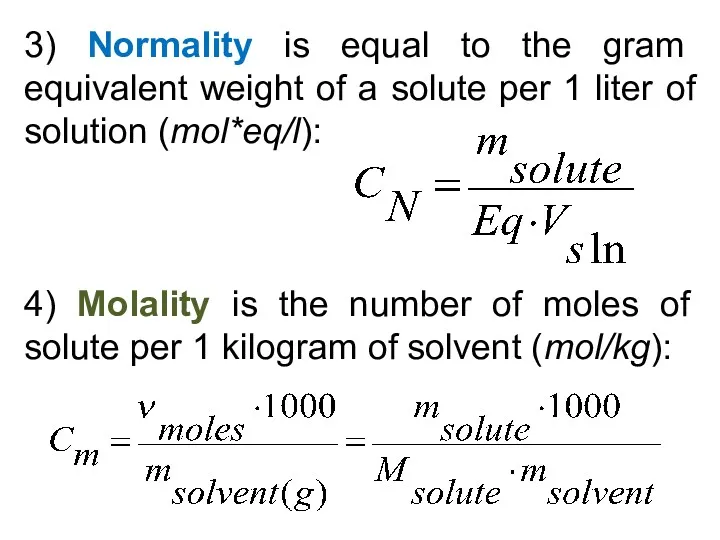
 Химические свойства кислот в свете теории электролитической диссоциации
Химические свойства кислот в свете теории электролитической диссоциации Расчет материального и теплового баланса процесса пиролиза гексана
Расчет материального и теплового баланса процесса пиролиза гексана Активационный анализ
Активационный анализ Бензол қатарындағы гетерофункционалды туындылары дәрі-дәрмек ретінде
Бензол қатарындағы гетерофункционалды туындылары дәрі-дәрмек ретінде Непредельные углеводороды
Непредельные углеводороды Моноядерні арени
Моноядерні арени Титриметрический метод анализа
Титриметрический метод анализа Основы коррозии и защиты металлов
Основы коррозии и защиты металлов Особенности дисперсных систем
Особенности дисперсных систем Решение задачи №4. Старость - на радость. Команда Карбораны
Решение задачи №4. Старость - на радость. Команда Карбораны Сплавы и коррозия металлов
Сплавы и коррозия металлов Основания. Значение оснований
Основания. Значение оснований Салыстырмалы тығыздығы мен элементтердің массалық үлестері бойынша газ күйіндегі заттардың молекулалық формулаларын табу
Салыстырмалы тығыздығы мен элементтердің массалық үлестері бойынша газ күйіндегі заттардың молекулалық формулаларын табу Дослідження методів очищення висококольорових поверхневих вод
Дослідження методів очищення висококольорових поверхневих вод Изменения, происходящие с белками в процессах технологической переработки сырья
Изменения, происходящие с белками в процессах технологической переработки сырья Свинец
Свинец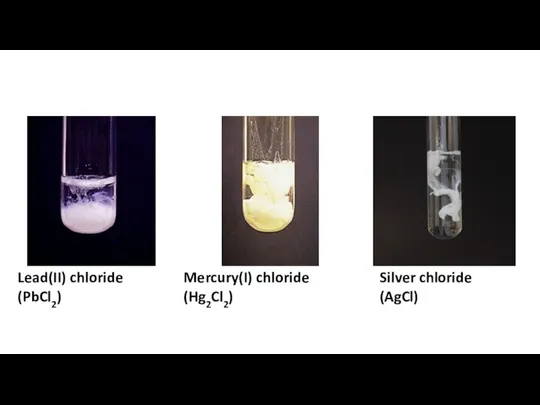 Separation amp confirmation
Separation amp confirmation Количественная характеристика растворов, растворение, растворимость
Количественная характеристика растворов, растворение, растворимость Тепловой эффект химической реакции
Тепловой эффект химической реакции Вода з точки зору хімії
Вода з точки зору хімії Циклоалкандар
Циклоалкандар Основи. Хімія
Основи. Хімія Металлы. Определения. Положение в периодической системе. Металлы в алхимии
Металлы. Определения. Положение в периодической системе. Металлы в алхимии Соли (12 класс)
Соли (12 класс) Крахмал. Строение вещества. Физические и химические свойства
Крахмал. Строение вещества. Физические и химические свойства Гидрокарбонат натрия NaHCO₃
Гидрокарбонат натрия NaHCO₃ Натуральный каучук
Натуральный каучук Требования к осадителю
Требования к осадителю