Elements 17 (7A) group. Study of the properties of halogens and the determination of halide ions in aqueous solution презентация
Содержание
- 2. Outline Introduction Main part 1. General characteristics of halogens 2. Chemical properties of halogens 3. Chlorine
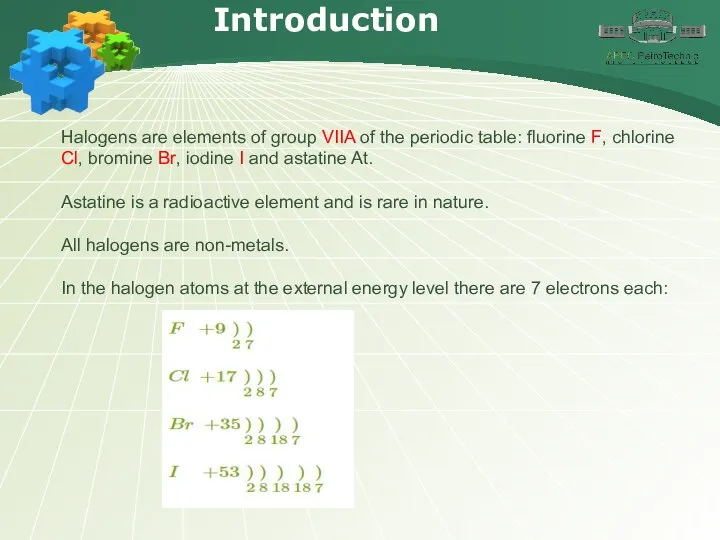
- 4. Introduction Halogens are elements of group VIIA of the periodic table: fluorine F, chlorine Cl, bromine
- 5. Introduction The valence electrons of the halogens form three electron pairs, and one electron of the
- 6. Introduction Fluorine has a higher electronegativity than other elements, and therefore the oxidation state –1 is
- 7. General characteristics of simple substances Halogen atoms combine in pairs and form diatomic molecules: F2, Cl2,
- 8. General characteristics of simple substances Fluorine Chlorine Bromine Iodine
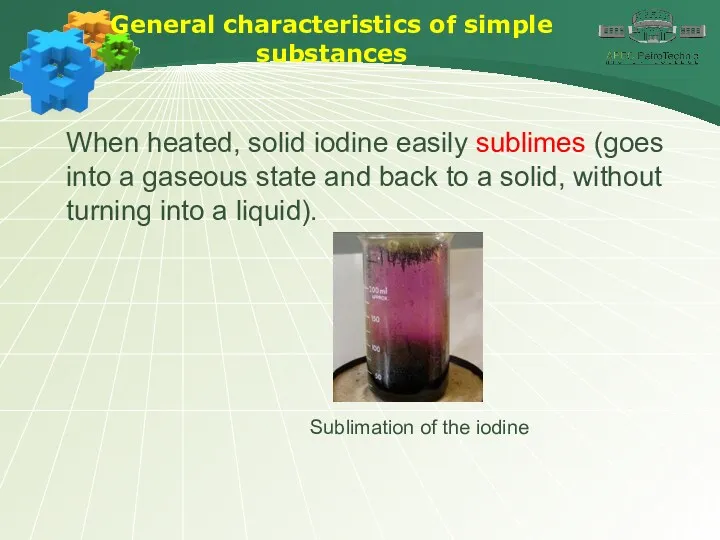
- 9. General characteristics of simple substances When heated, solid iodine easily sublimes (goes into a gaseous state
- 10. General characteristics of simple substances All halogens have a strong, unpleasant odor and are highly toxic.
- 11. 2. Chemical properties of halogens Halogens are reactive substances. In reactions with metals and most non-metals,
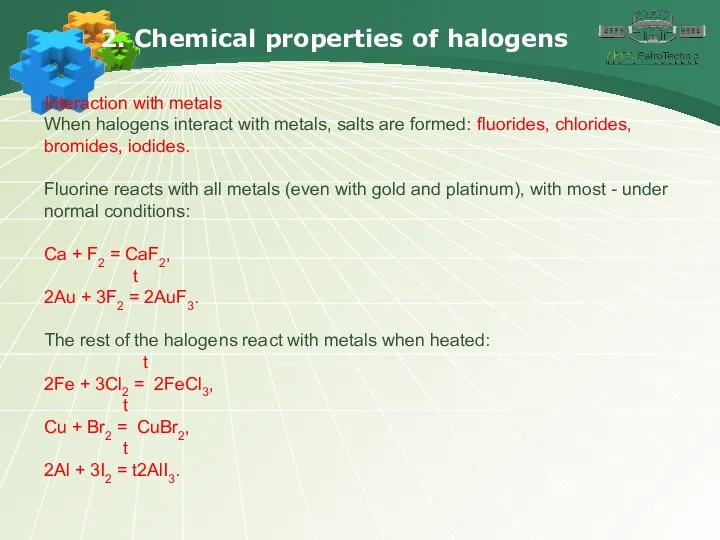
- 12. 2. Chemical properties of halogens Interaction with metals When halogens interact with metals, salts are formed:
- 13. 2. Chemical properties of halogens Interaction with hydrogen In the reactions of halogens with hydrogen, gaseous
- 14. 2. Chemical properties of halogens The reaction of iodine with hydrogen is slow, even when heated.
- 15. 2. Chemical properties of halogens The each other displacements of Halogens from salts In the reactions
- 16. 2. Chemical properties of halogens Bromine is able to displace iodine from iodides, but does not
- 17. 3. Chlorine and its compounds Chlorine Chlorine is a poisonous, yellow-green gas with an unpleasant odor.
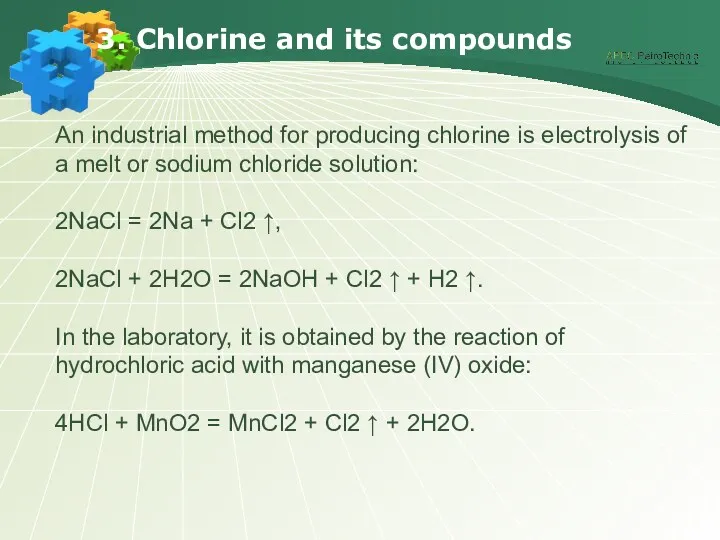
- 18. 3. Chlorine and its compounds An industrial method for producing chlorine is electrolysis of a melt
- 19. 3. Chlorine and its compounds Hydrogen chloride Hydrogen chloride is formed by the interaction of chlorine
- 20. 3. Chlorine and its compounds Hydrochloric acid A solution of hydrogen chloride in water is called
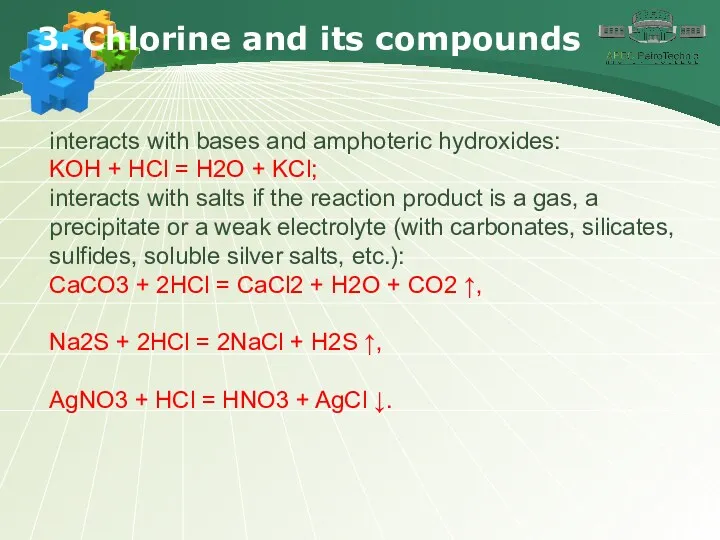
- 21. 3. Chlorine and its compounds interacts with bases and amphoteric hydroxides: KOH + HCl = H2O
- 22. 3. Chlorine and its compounds Chlorides Most hydrochloric acid salts are readily soluble in water. Silver
- 23. 4. Halogens in nature. The use of halogens and their compounds Halogens in nature Halogens are
- 24. 4. Halogens in nature. The use of halogens and their compounds The most common chlorine compounds
- 25. 4. Halogens in nature. The use of halogens and their compounds Bromine and iodine do not
- 26. 4. Halogens in nature. The use of halogens and their compounds Halogens in living organisms All
- 27. 4. Halogens in nature. The use of halogens and their compounds Bromine compounds regulate the processes
- 28. 4. Halogens in nature. The use of halogens and their compounds The use of halogens and
- 29. 4. Halogens in nature. The use of halogens and their compounds Molecular chlorine is used for
- 30. 4. Halogens in nature. The use of halogens and their compounds Table salt is added to
- 31. 1.Cl2 reacts with substance (s): A)CuCl2 B)LiI C)CuBr2 D)FeF3 2.Note the property of hydrogen chloride: A)forms
- 32. 4.In the series F - Cl - Br - I, it decreases: A)number of protons in
- 33. 8.All substances of the series enter into a reaction with hydrochloric acid: A)Ag, Cu, Au B)Ca(OH)2,
- 34. Literature 1.Basic literature : 1. Jenkins, Chemistry, ISBN 978-0-17-628930-0 2. Alberta Learning, Chemistry data booklet 2010,
- 35. 2.Additional literature : 1.Б.А.Мансуров «Химия» 10-11 кл., Атамура 2015 г 2.Б.Мансуров., Н.Торшина «Методика преподавания органической химии»
- 37. Скачать презентацию




























 ОВР в органической химии
ОВР в органической химии Ферментативный катализ, влияние давления, концентрации и катализаторов на скорость химической реакции
Ферментативный катализ, влияние давления, концентрации и катализаторов на скорость химической реакции Кристалічна ґрадка. Встановити взаємозв’язок між будовою речовин та їх фізичними властивостями
Кристалічна ґрадка. Встановити взаємозв’язок між будовою речовин та їх фізичними властивостями Радиогеохимия метаморфических процессов
Радиогеохимия метаморфических процессов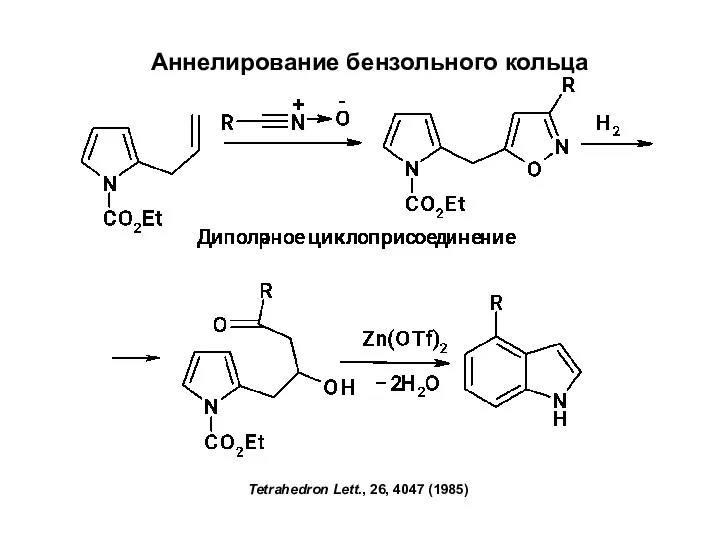 Аннелирование бензольного кольца
Аннелирование бензольного кольца Виведення молекулярної формули речовини за загальною формулою гомологічного ряду та густиною або відносною густиною
Виведення молекулярної формули речовини за загальною формулою гомологічного ряду та густиною або відносною густиною Азотная кислота. Получение, свойства. Нитраты, азотные удобрения
Азотная кислота. Получение, свойства. Нитраты, азотные удобрения Окислительно-восстановительное титрование. Перманганатометрия. Кривые титрования, ошибки
Окислительно-восстановительное титрование. Перманганатометрия. Кривые титрования, ошибки Задачи на смеси и сплавы. Метод Пирсона
Задачи на смеси и сплавы. Метод Пирсона Хімія та обмін вуглеводів
Хімія та обмін вуглеводів Алюминий и его соединения
Алюминий и его соединения Выращивание кристаллов методом Чохральского
Выращивание кристаллов методом Чохральского Супутній нафтовий газ
Супутній нафтовий газ Тағамдық және биологиялық белсенді қоспалар туралы жалпы мағлұматтар
Тағамдық және биологиялық белсенді қоспалар туралы жалпы мағлұматтар Соли, как производные кислот и оснований. Их состав и номенклатура
Соли, как производные кислот и оснований. Их состав и номенклатура Сахар - вред или польза?
Сахар - вред или польза? Алюминий и его соединения
Алюминий и его соединения Жесткость воды. Способы ее устранения
Жесткость воды. Способы ее устранения Объёмная доля компонента смеси
Объёмная доля компонента смеси МОЛЯРНЫЙ ОБЪЕМ Химия 8 класс
МОЛЯРНЫЙ ОБЪЕМ Химия 8 класс Кислородсодержащие органические соединения. Спирты
Кислородсодержащие органические соединения. Спирты Различные теории кислот и оснований
Различные теории кислот и оснований Прочность полимеров
Прочность полимеров Оксиды и гидроксиды
Оксиды и гидроксиды Оксид углерода-С
Оксид углерода-С Моделирование структуры биомакромолекул
Моделирование структуры биомакромолекул Вещества молекулярного и немолекулярного строения
Вещества молекулярного и немолекулярного строения Физико-химические процессы в тропосфере. Температурные инверсии. Смог в атмосфере городов. Аэрозоли
Физико-химические процессы в тропосфере. Температурные инверсии. Смог в атмосфере городов. Аэрозоли