Содержание
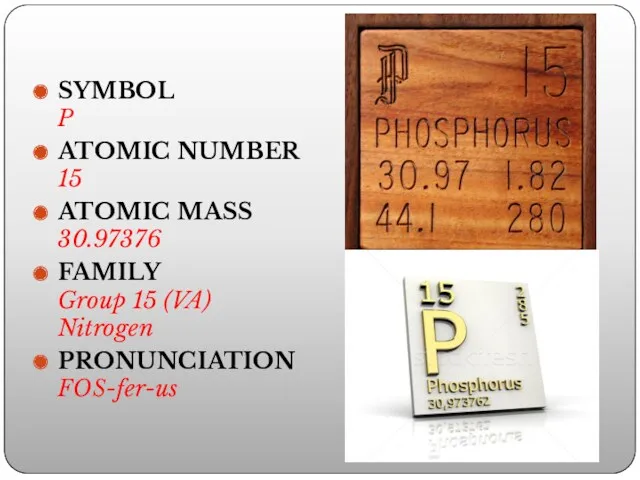
- 2. SYMBOL P ATOMIC NUMBER 15 ATOMIC MASS 30.97376 FAMILY Group 15 (VA) Nitrogen PRONUNCIATION FOS-fer-us
- 3. Phosphorus is element 15 on the periodic table, with the element symbol P. Because it is
- 4. DISCOVERY Phosphorus was discovered in 1669 by Hennig Brand in Germany. Brand isolated phosphorus from urine.
- 5. PHYSICAL PROPERTIES OF PHOSPHORUS
- 6. White phosphorus is a waxy, transparent solid. Its melting point is 44.1°C (111°F) and its boiling
- 7. RED PHOSPHORUS Red phosphorus is a red powder. It can be made by heating white phosphorus
- 8. BLACK PHOSPHORUS Black phosphorus looks like graphite powder. Graphite is a form of carbon used in
- 9. CHEMICAL PROPERTIES Red and black phosphorus is obtained from white. White phosphorus is obtained by reducing
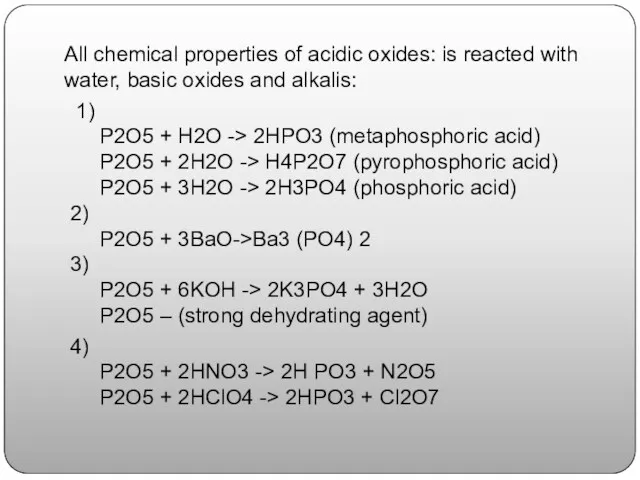
- 10. All chemical properties of acidic oxides: is reacted with water, basic oxides and alkalis: 1) P2O5
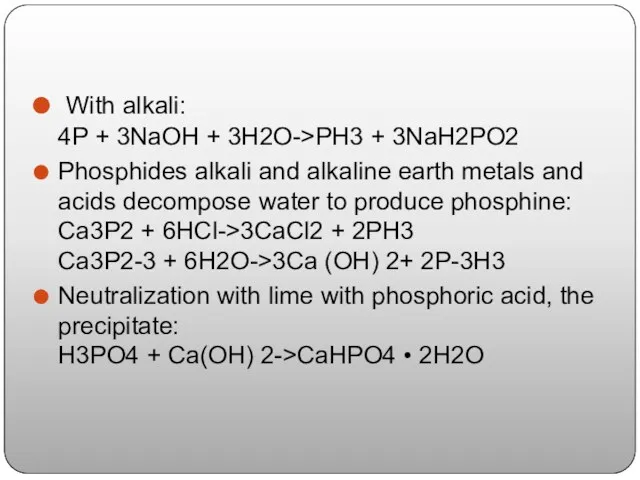
- 11. With alkali: 4P + 3NaOH + 3H2O->PH3 + 3NaH2PO2 Phosphides alkali and alkaline earth metals and
- 12. COMPOUNDS H3PO4 – orthophosphoric acid H3PO3 – phosphorous acid H4P2O6 – hypophosphoric acid H3PO2 – hypophosphorous
- 13. ISOTOPES Phosphorus has 22 known isotopes. P-31 is the only stable isotope. Six radioactive isotopes of
- 14. OCCURRENCE Because of its chemical activity phosphorus does not occur uncombined in nature but is widely
- 15. PRODUCTION About 1,000,000 short tons (910,000 t) of elemental phosphorus is produced annually. Calcium phosphate (phosphate
- 16. USES OF PHOSPHORUS Red phosphorus, which is relatively stable, is used to make safety matches, tracer
- 19. Bone ash (calcium phosphate) is used to make chinaware and to make monocalcium phosphate for baking
- 22. Скачать презентацию















 Спектрофометрия
Спектрофометрия Характеристика s,p,d,f - элементов
Характеристика s,p,d,f - элементов Химическое кафе Кислоты
Химическое кафе Кислоты Експлуатаційні матеріали. Пальне для карбюраторних, дизельних двигунів. Змащувальні масла, пластичні мастила військової техніки
Експлуатаційні матеріали. Пальне для карбюраторних, дизельних двигунів. Змащувальні масла, пластичні мастила військової техніки Фосфор и его соединения. Электронные формулы атома фосфора
Фосфор и его соединения. Электронные формулы атома фосфора Тепловой эффект химических реакций. 8 класс
Тепловой эффект химических реакций. 8 класс Химия углеводов
Химия углеводов Пластмаси та їх роль у сучасному виробництві
Пластмаси та їх роль у сучасному виробництві Количество вещества, число Авогадро, молярная масса, молярный объём, уравнение связи
Количество вещества, число Авогадро, молярная масса, молярный объём, уравнение связи Основи. Властивості, застосування гідроксидів Натрію і Калію
Основи. Властивості, застосування гідроксидів Натрію і Калію Интересные факты о химических веществах
Интересные факты о химических веществах Observing change. Chemical reactions
Observing change. Chemical reactions Группа веществ, изолируемых полярными растворителями
Группа веществ, изолируемых полярными растворителями Периодическая система химических элементов Д.И. Менделеева. Знаки химических элементов
Периодическая система химических элементов Д.И. Менделеева. Знаки химических элементов Полимеры. Полимерные материалы
Полимеры. Полимерные материалы Crystal defects
Crystal defects Валентность химических элементов (8 класс)
Валентность химических элементов (8 класс) Диеновые углеводороды или алкадиены (тема 4)
Диеновые углеводороды или алкадиены (тема 4) Milk Composition. Proteins - Молоко
Milk Composition. Proteins - Молоко Капиллярная конденсация
Капиллярная конденсация Дисахаридтер. Сахароза
Дисахаридтер. Сахароза Періодична система хімічних елементів. Хімія. 8 клас
Періодична система хімічних елементів. Хімія. 8 клас Щелочные металлы
Щелочные металлы Альдегиды и кетоны
Альдегиды и кетоны Изучение упругости диссоциации карбоната кальция
Изучение упругости диссоциации карбоната кальция Дикарбоновые , гидроксикислоты
Дикарбоновые , гидроксикислоты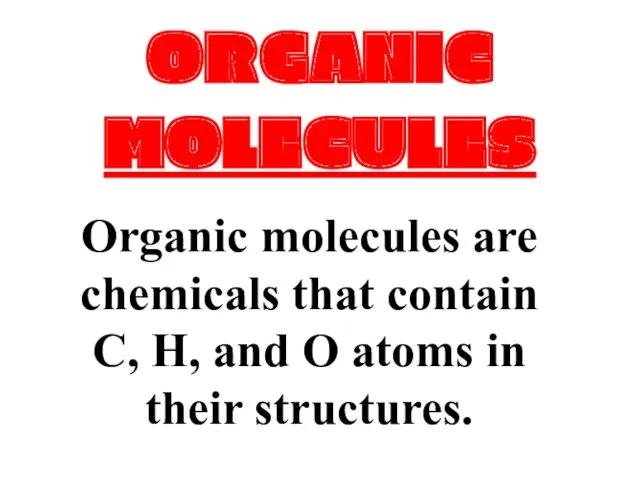 Organic molecules
Organic molecules Обмен липидов
Обмен липидов