Содержание
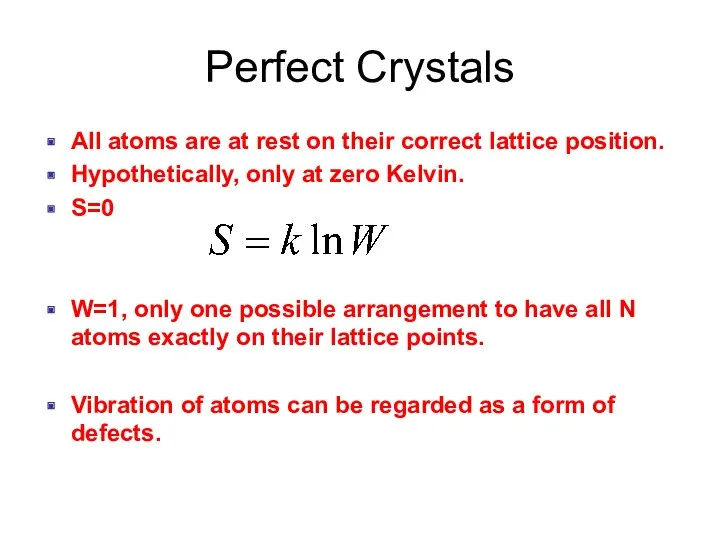
- 2. Perfect Crystals All atoms are at rest on their correct lattice position. Hypothetically, only at zero
- 3. Classification of defects in solids Zero-dimensional (point) defects Vacancies, Interstitial atoms (ions), Foreign atoms (ions) One-dimensional
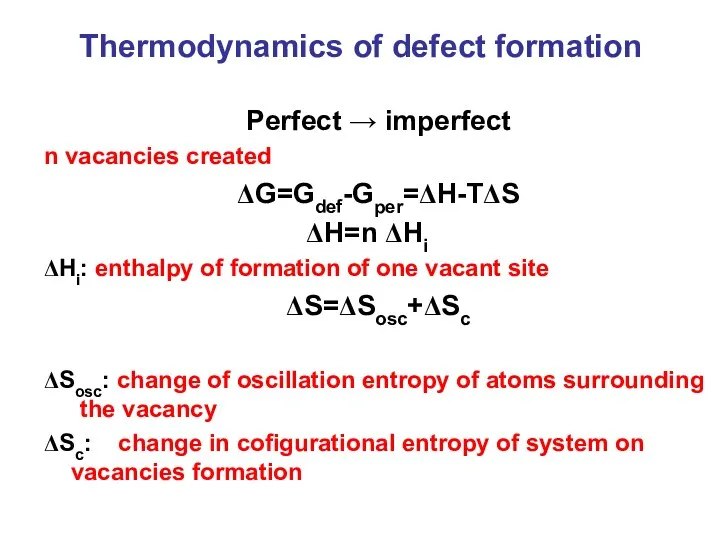
- 4. Thermodynamics of defect formation Perfect → imperfect n vacancies created ΔG=Gdef-Gper=ΔH-TΔS ΔH=n ΔHi ΔHi: enthalpy of
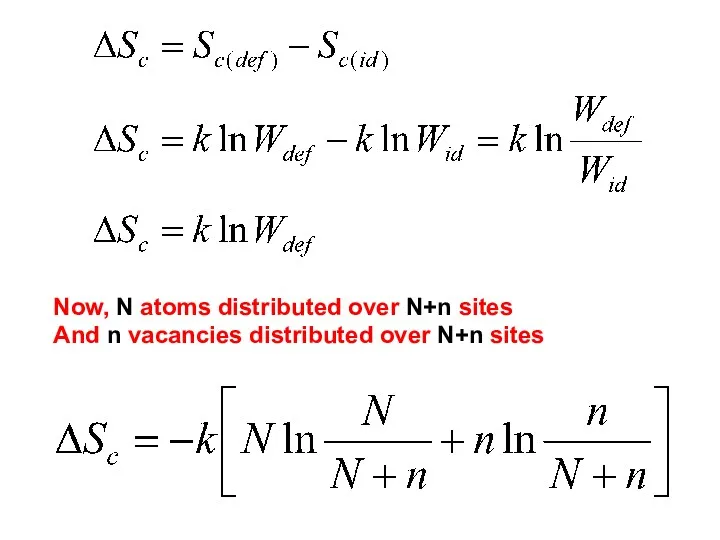
- 5. Now, N atoms distributed over N+n sites And n vacancies distributed over N+n sites
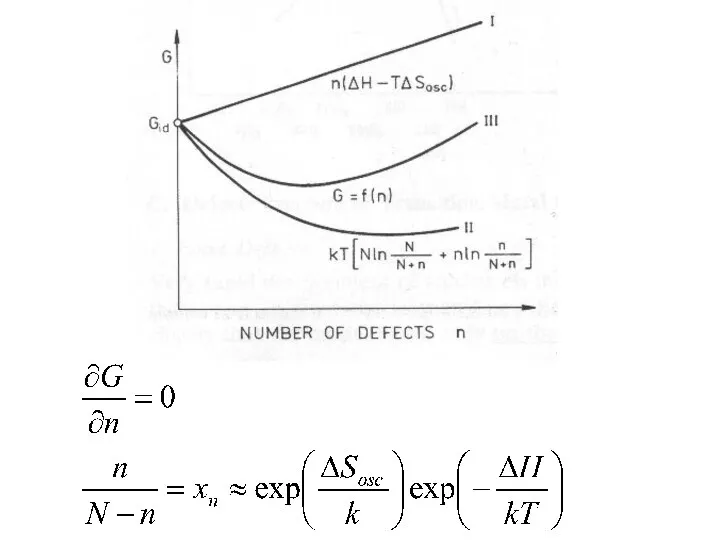
- 6. ΔH always positive ΔSosc always negative n/(N+n)
- 8. Defect formation possible only due to increased configurational entropy in that process. After n exceeds a
- 9. Crystal Defects Defects can affect Strength Conductivity Deformation style Color
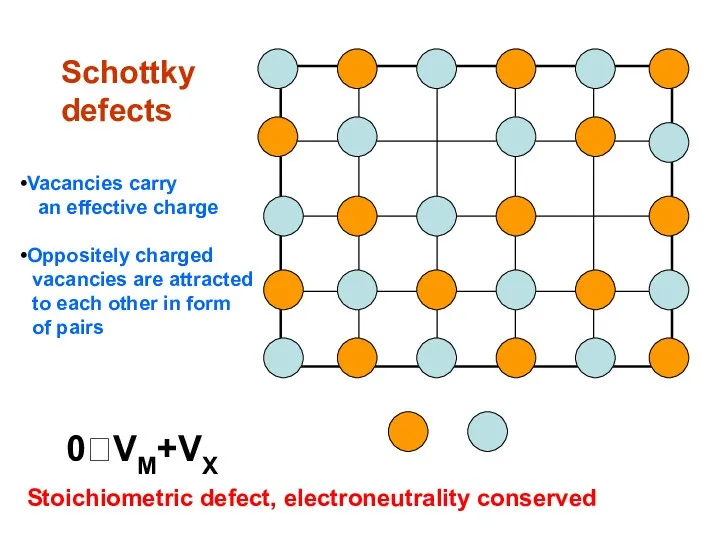
- 10. Schottky defects 0⮀VM+VX Stoichiometric defect, electroneutrality conserved Vacancies carry an effective charge Oppositely charged vacancies are
- 11. NaCl Dissociation enthalpy for vacancies pairs ≈ 120 kJ/mol. At room temperature, 1 of 1015 crystal
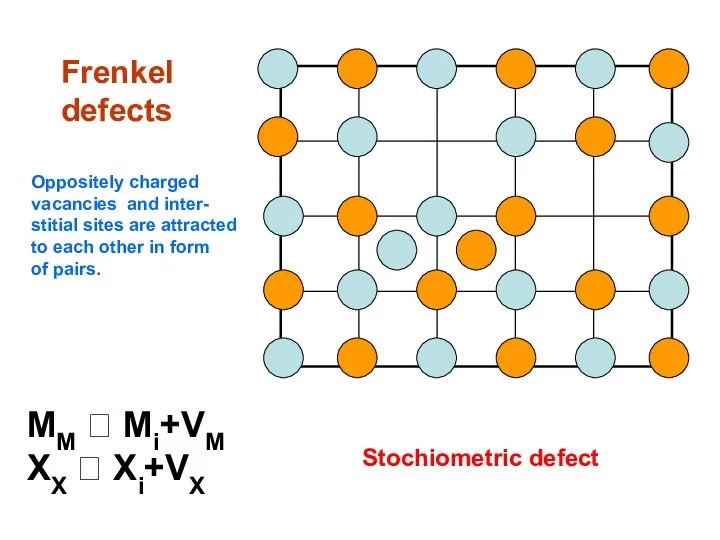
- 12. Frenkel defects MM ⮀ Mi+VM XX ⮀ Xi+VX Stochiometric defect Oppositely charged vacancies and inter- stitial
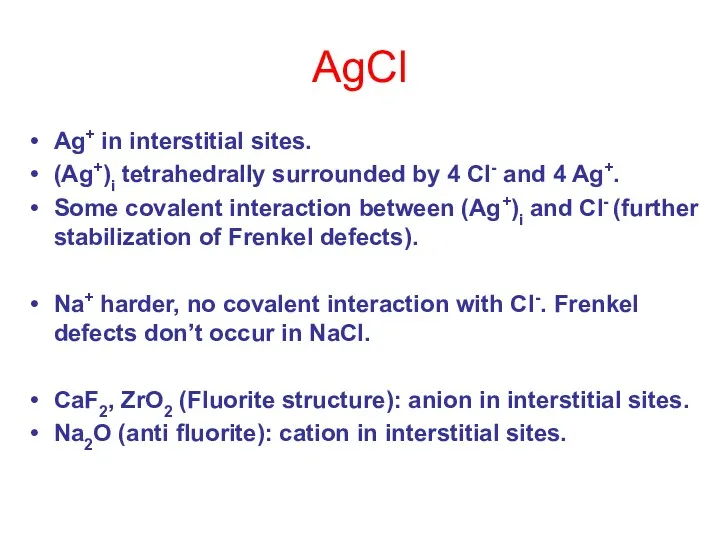
- 13. AgCl Ag+ in interstitial sites. (Ag+)i tetrahedrally surrounded by 4 Cl- and 4 Ag+. Some covalent
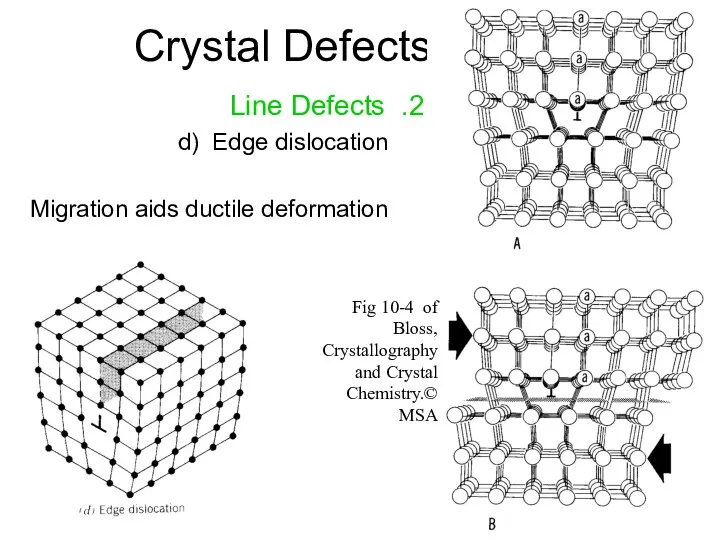
- 14. Crystal Defects 2. Line Defects d) Edge dislocation Migration aids ductile deformation Fig 10-4 of Bloss,
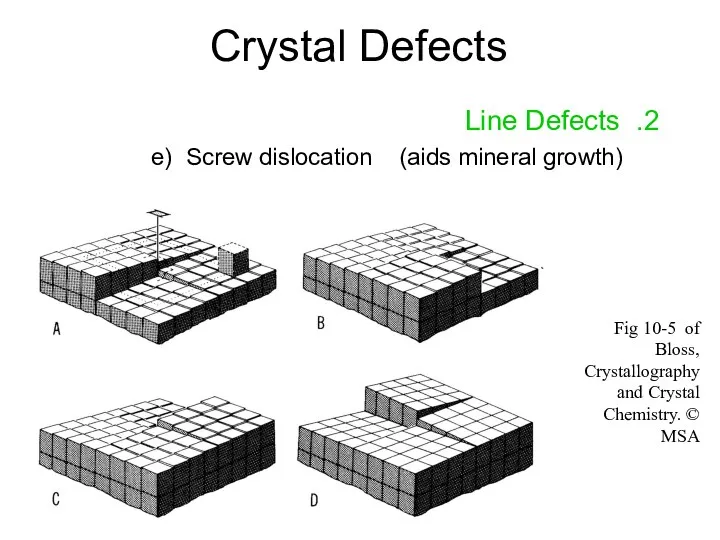
- 15. Crystal Defects 2. Line Defects e) Screw dislocation (aids mineral growth) Fig 10-5 of Bloss, Crystallography
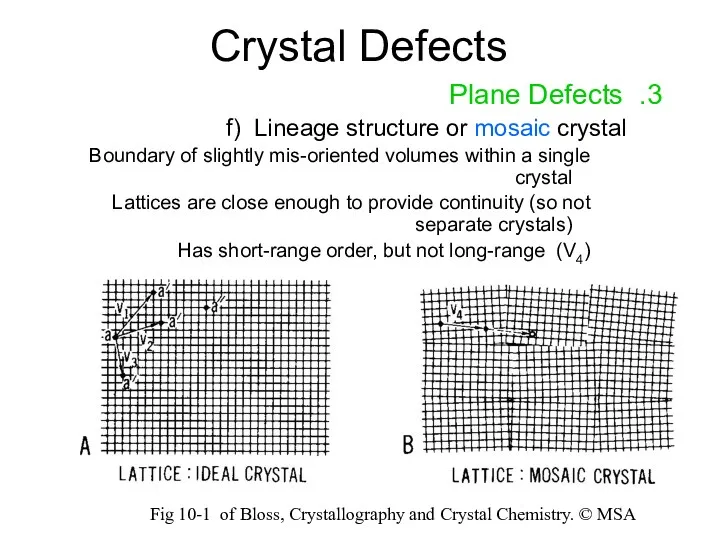
- 16. Crystal Defects 3. Plane Defects f) Lineage structure or mosaic crystal Boundary of slightly mis-oriented volumes
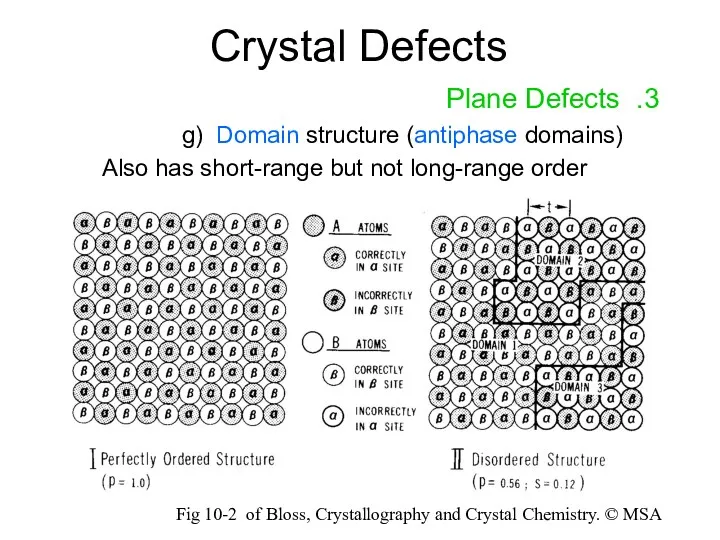
- 17. Crystal Defects 3. Plane Defects g) Domain structure (antiphase domains) Also has short-range but not long-range
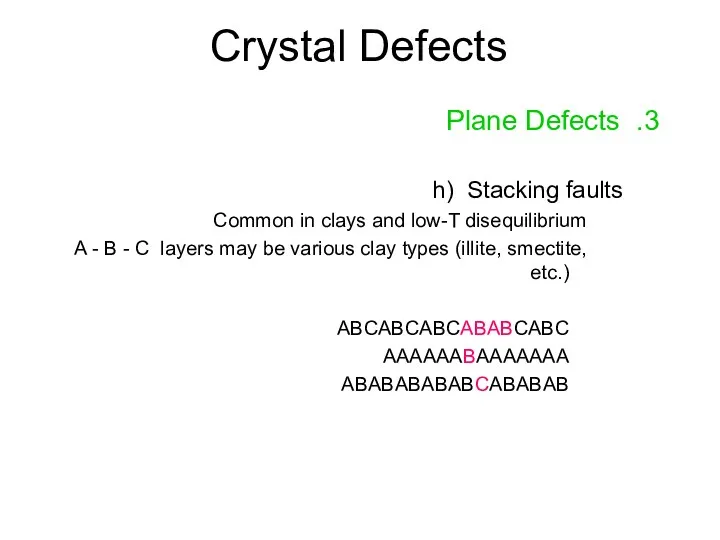
- 18. Crystal Defects 3. Plane Defects h) Stacking faults Common in clays and low-T disequilibrium A -
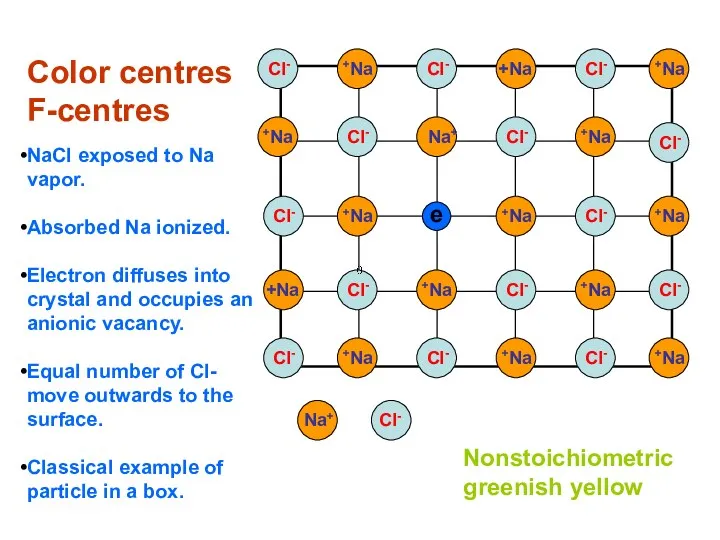
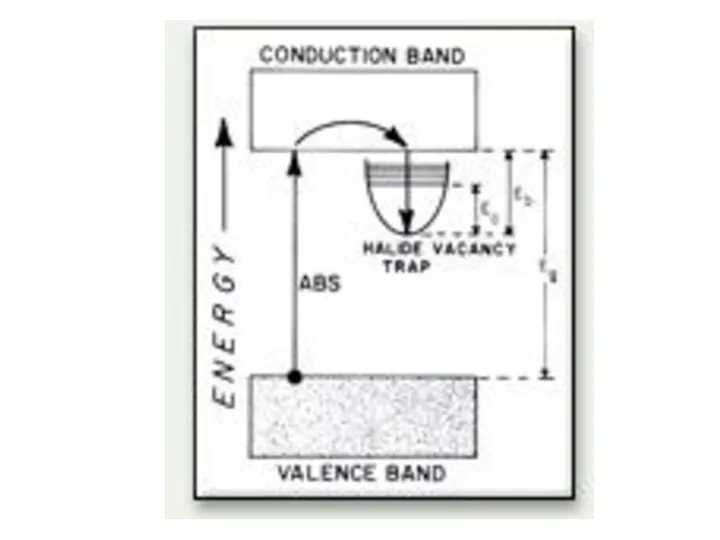
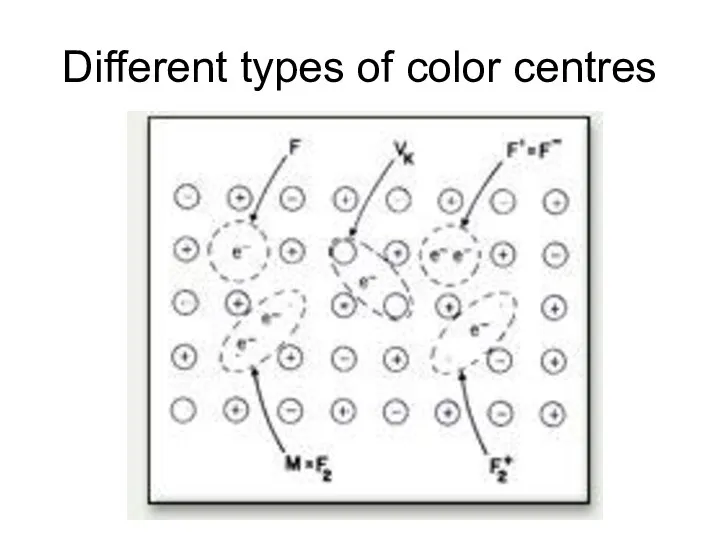
- 19. Color centres F-centres NaCl exposed to Na vapor. Absorbed Na ionized. Electron diffuses into crystal and
- 20. Color depends on host crystal not on nature of vapor. K vapors would produce the same
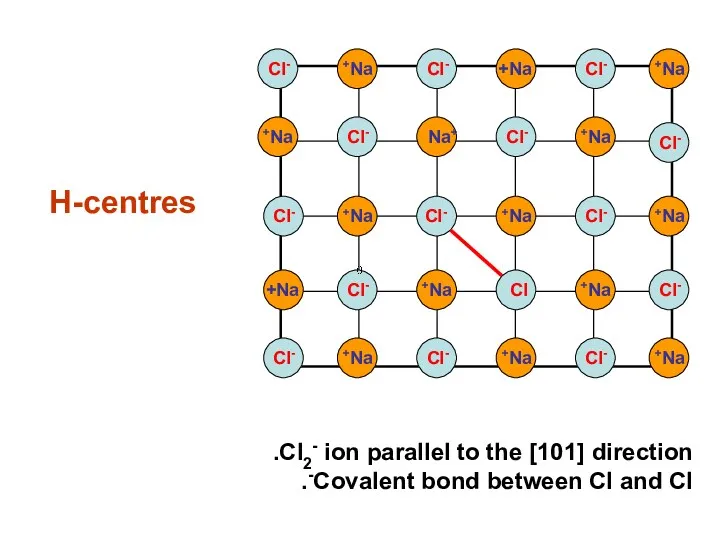
- 22. H-centres Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+
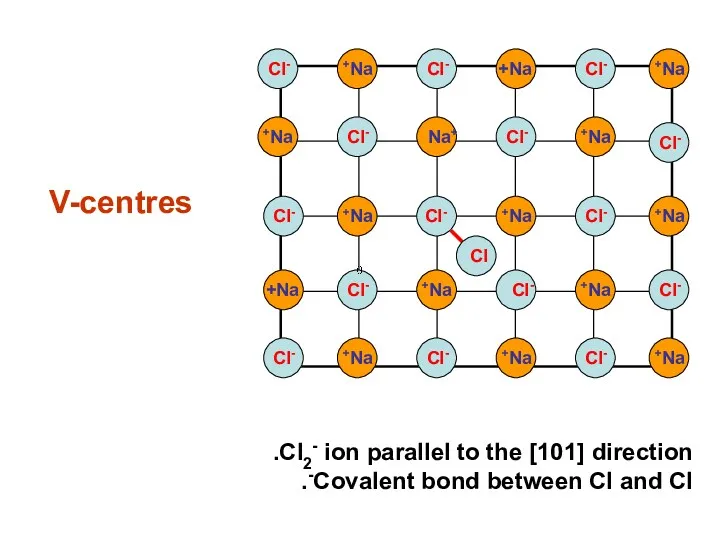
- 23. V-centres Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+
- 24. Different types of color centres
- 25. Colors in the solid state
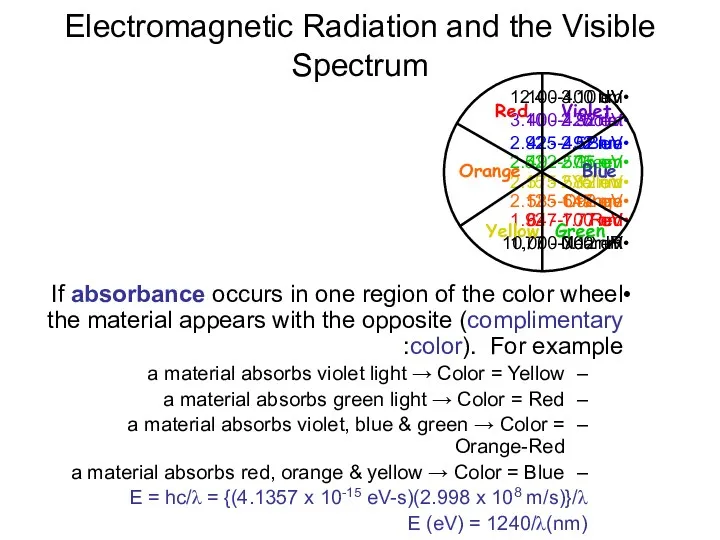
- 26. Electromagnetic Radiation and the Visible Spectrum UV 100-400 nm 12.4 - 3.10 eV Violet 400-425 nm
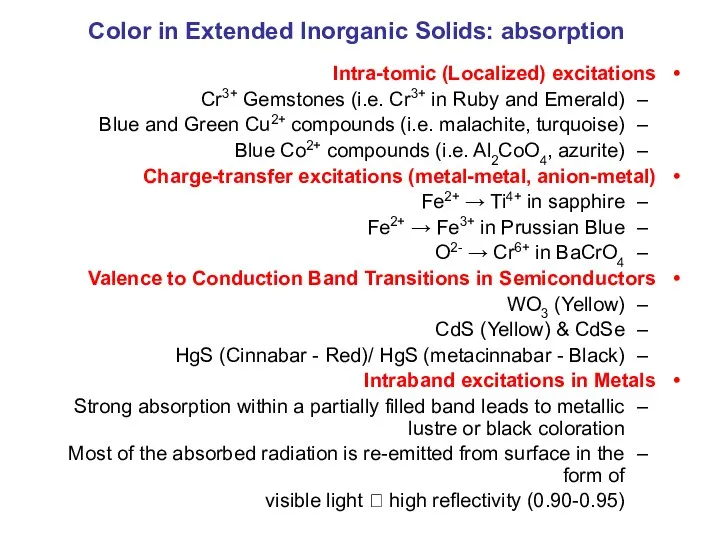
- 27. Color in Extended Inorganic Solids: absorption Intra-tomic (Localized) excitations Cr3+ Gemstones (i.e. Cr3+ in Ruby and
- 28. Gemstones
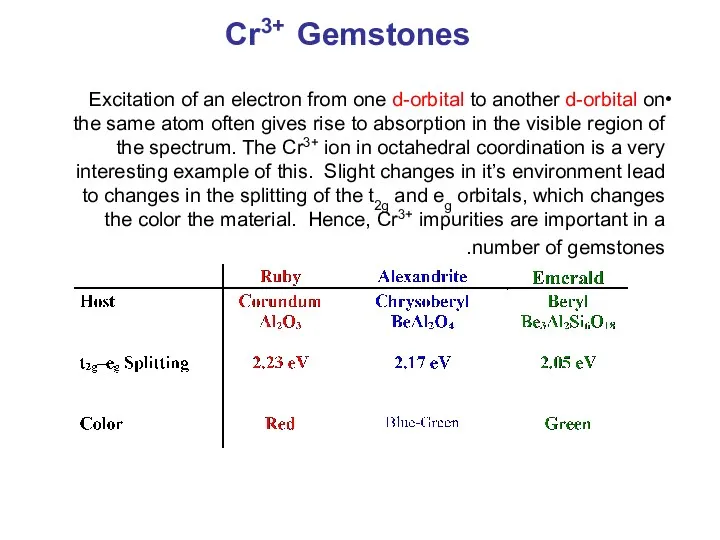
- 29. Cr3+ Gemstones Excitation of an electron from one d-orbital to another d-orbital on the same atom
- 30. Red ruby. The name ruby comes from the Latin "Rubrum" meaning red. The ruby is in
- 31. Green emerald. The mineral is transparent emerald, the green variety of Beryl on calcite matrix. 2.5
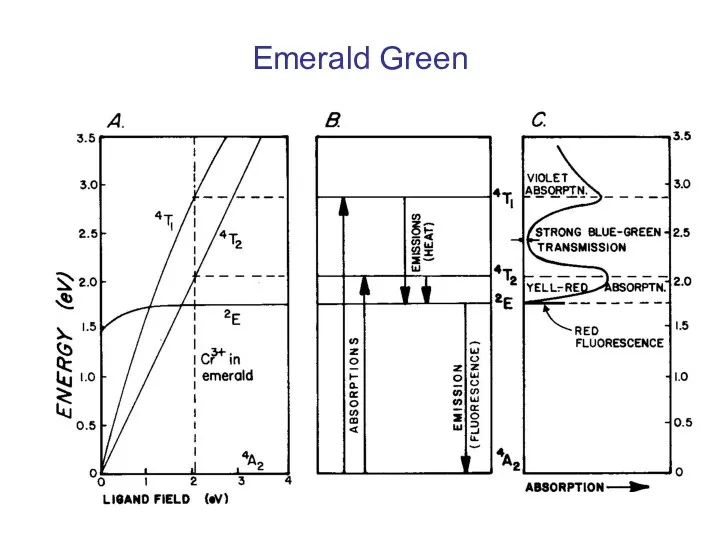
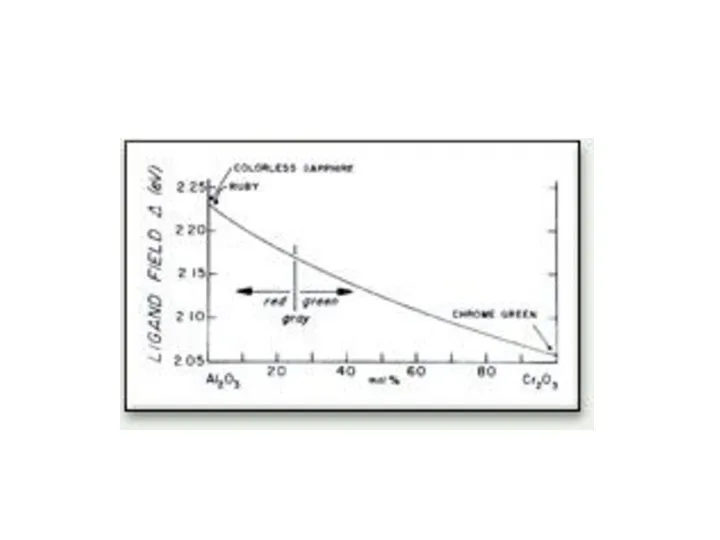
- 32. Tunabe-Sugano Diagram Cr3+ The Tunabe-Sugano diagram below shows the allowed electronic excitations for Cr3+ in an
- 33. Ruby Red
- 34. Emerald Green
- 35. A synthetic alexandrite gemstone, 5 mm across, changing from a reddish color in the light from
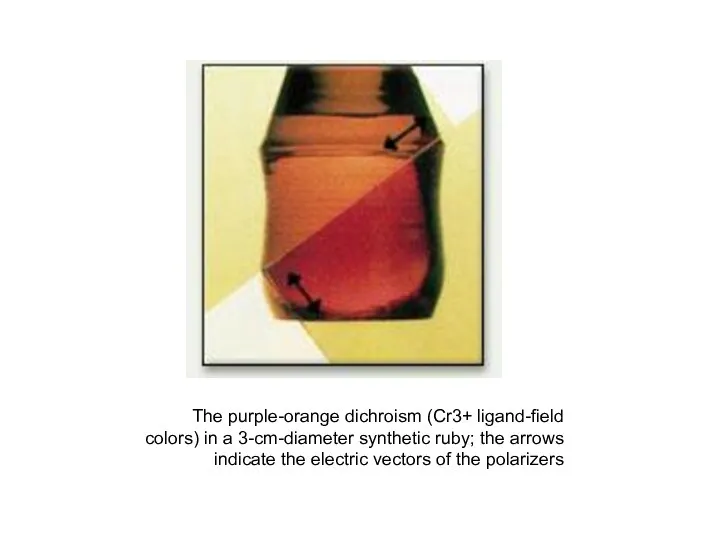
- 37. The purple-orange dichroism (Cr3+ ligand-field colors) in a 3-cm-diameter synthetic ruby; the arrows indicate the electric
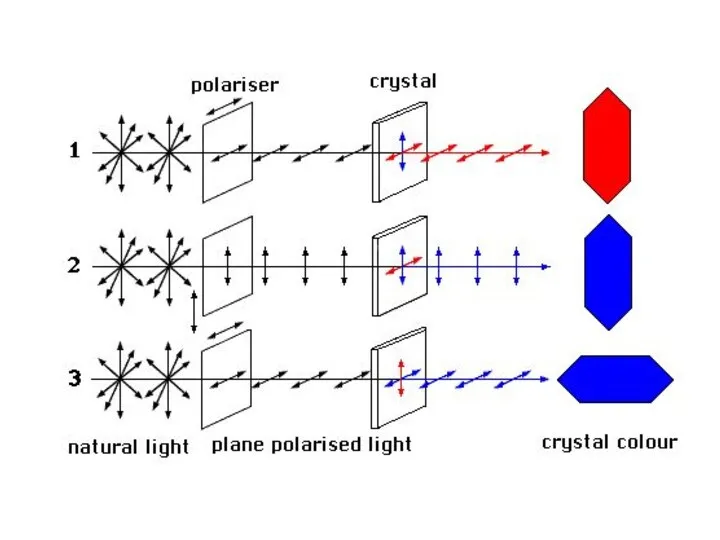
- 38. Pleochroism is the ability of a mineral to absorb different wavelengths of transmitted light depending upon
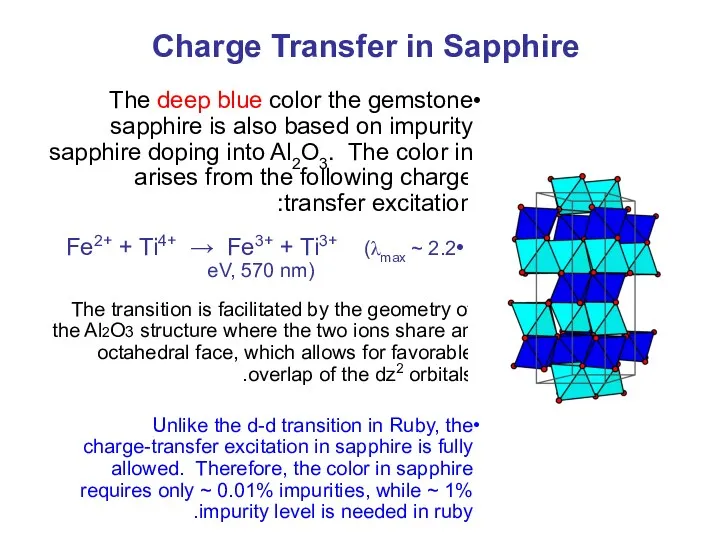
- 40. Charge Transfer in Sapphire The deep blue color the gemstone sapphire is also based on impurity
- 41. In magnetite, the black iron oxide Fe3O4 or Fe2+O . Fe3+2O3, there is "homonuclear" charge transfer
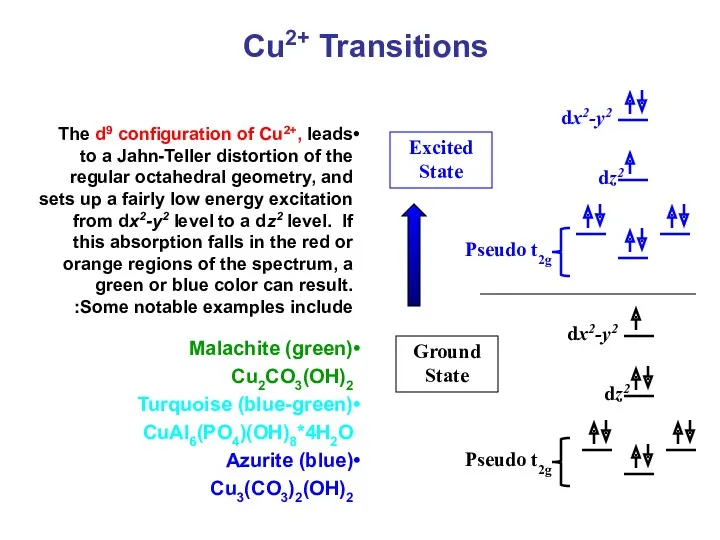
- 42. Cu2+ Transitions The d9 configuration of Cu2+, leads to a Jahn-Teller distortion of the regular octahedral
- 44. Скачать презентацию















 Химическое равновесие. Принцип Ле Шателье
Химическое равновесие. Принцип Ле Шателье Соединения галогенов
Соединения галогенов Аминокислоты. Пептиды. Хроматографические методы исследования
Аминокислоты. Пептиды. Хроматографические методы исследования Химическая связь
Химическая связь Массовая доля вещества в растворе
Массовая доля вещества в растворе Визначення іонів лужних і лужноземельних іонів у природних водах
Визначення іонів лужних і лужноземельних іонів у природних водах Уникальная соль (для дошколников)
Уникальная соль (для дошколников) Пищевые добавки
Пищевые добавки Коллигативные свойства растворов
Коллигативные свойства растворов Основы термической и химико-термической обработки стали. Теория и технология термической обработки стали. Лекция 3. Тема 7
Основы термической и химико-термической обработки стали. Теория и технология термической обработки стали. Лекция 3. Тема 7 Литий. Физические свойства лития
Литий. Физические свойства лития Типы заданий. ЕГЭ №32
Типы заданий. ЕГЭ №32 20230419_izomery
20230419_izomery Растворы и растворители
Растворы и растворители Фосфор и его соединения
Фосфор и его соединения Общая характеристика неметаллов
Общая характеристика неметаллов Простые вещества. Игра Счастливый случай
Простые вещества. Игра Счастливый случай Элемент, имеющий относительную атомную массу
Элемент, имеющий относительную атомную массу Введение в аналитическую химию. Введение в качественный анализ
Введение в аналитическую химию. Введение в качественный анализ Алканы. Гомологи
Алканы. Гомологи Методы разделения и исследования состава нефти и газа
Методы разделения и исследования состава нефти и газа Формальная кинетика. Предмет химической кинетики
Формальная кинетика. Предмет химической кинетики Хром. Элемент под № 24
Хром. Элемент под № 24 Аммиак. Состав вещества
Аммиак. Состав вещества Галогены. Фтор, хлор, бром, йод, астат
Галогены. Фтор, хлор, бром, йод, астат Породообразующие минералы
Породообразующие минералы Особенности строения, реакционной способности и методы синтеза гидроксилсодержащих соединений
Особенности строения, реакционной способности и методы синтеза гидроксилсодержащих соединений Взрывоопасные грузы
Взрывоопасные грузы