Слайд 2

Physical change
These are physical changes where there is no change in
particles, just their arrangement and their energy.
Слайд 3

Chemical change
These are examples of chemical changes where a chemical reaction
takes place and a new substance is formed. During a chemical change energy may be released or absorbed.
Слайд 4

Chemical reactions
During chemical reactions the atoms (particles) rearrange to form a
new substance.
The signs that indicate that this has occurred are:
colour change
light is emitted
change in temperature
bubbles of gas are produced.
Слайд 5
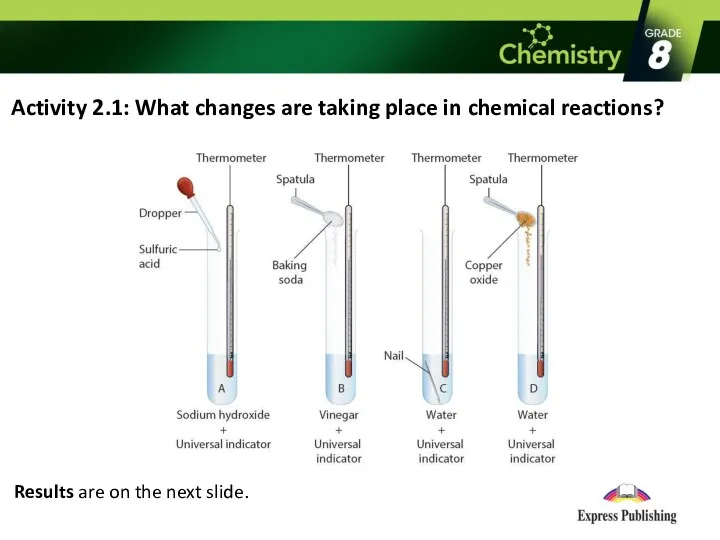
Activity 2.1: What changes are taking place in chemical reactions?
Results are
on the next slide.
Слайд 6
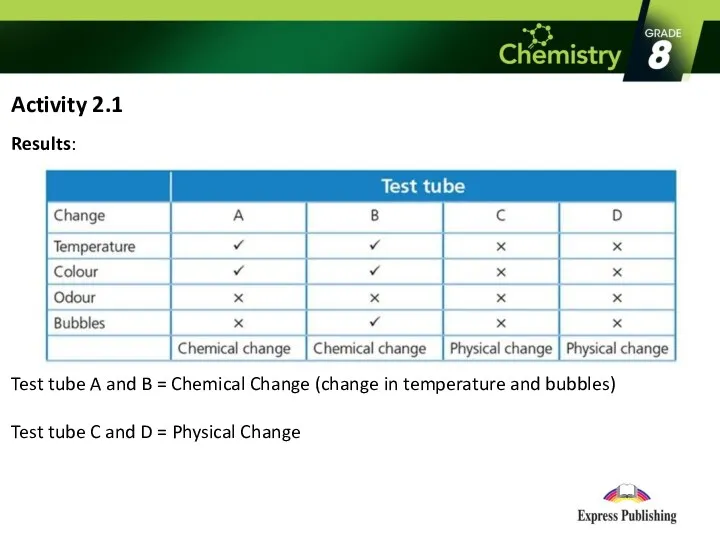
Activity 2.1
Results:
Test tube A and B = Chemical Change (change
in temperature and bubbles)
Test tube C and D = Physical Change
Слайд 7
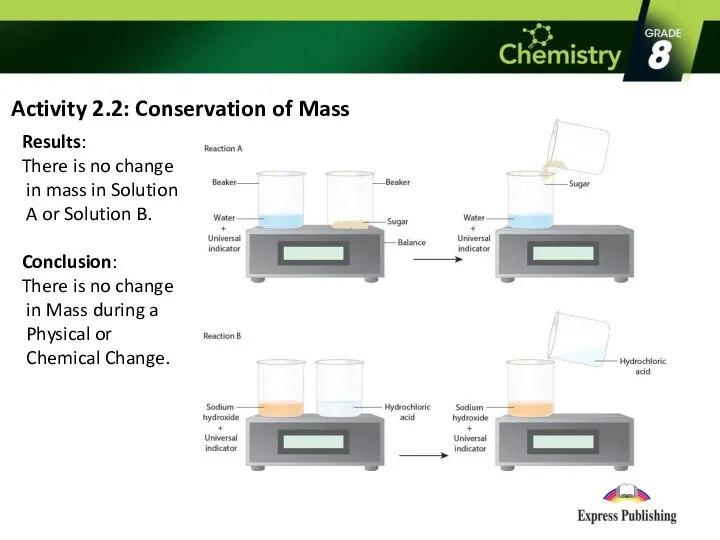
Activity 2.2: Conservation of Mass
Results:
There is no change in
mass in Solution A or Solution B.
Conclusion:
There is no change in Mass during a Physical or Chemical Change.
Слайд 8
Law of Conservation of Mass
Antoine Lavoisier discovered that the mass
of a substance cannot be created or destroyed, so during a physical and chemical change there is no change in the overall mass.
Слайд 9
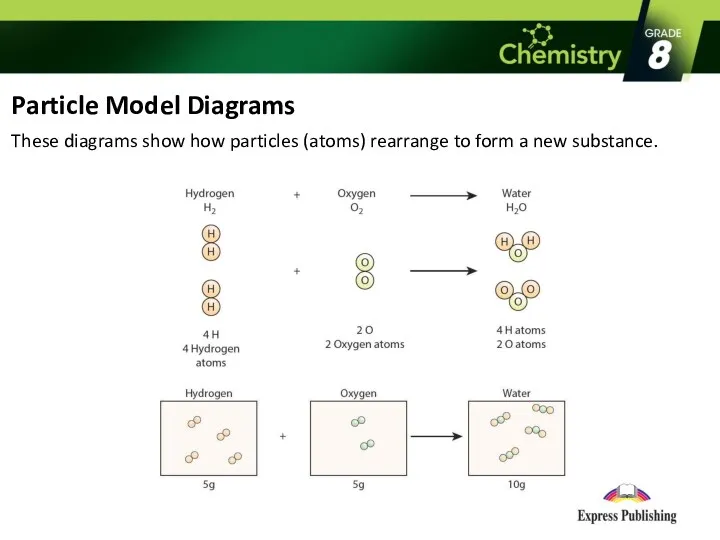
Particle Model Diagrams
These diagrams show how particles (atoms) rearrange to form
a new substance.
Слайд 10
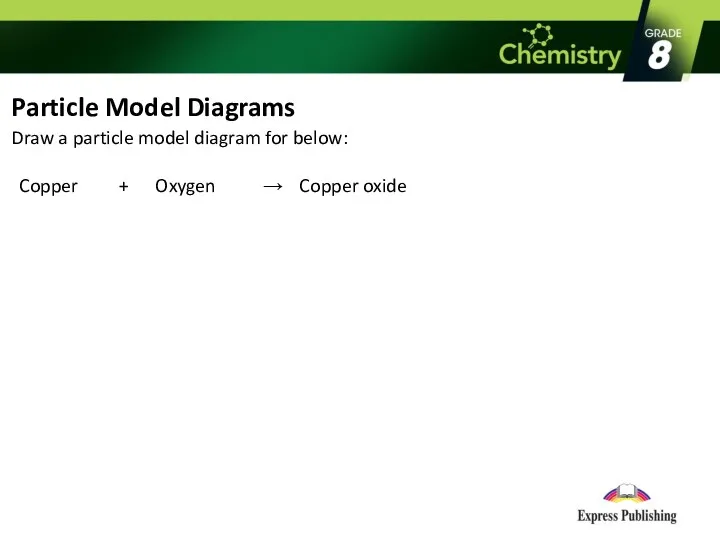
Particle Model Diagrams
Draw a particle model diagram for below:
Copper + Oxygen → Copper oxide









 Повторение и обобщение знаний по темам Металлы и сплавы.
Повторение и обобщение знаний по темам Металлы и сплавы.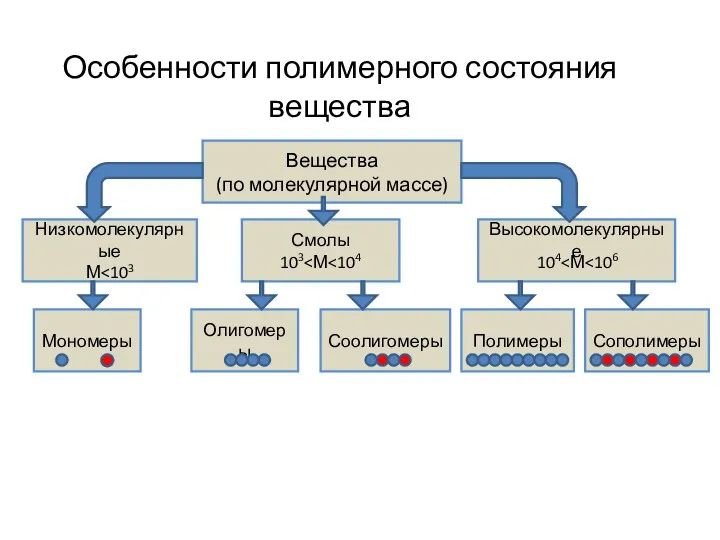 Особенности полимерного состояния вещества
Особенности полимерного состояния вещества Тағамдық қоспалардың функционалдық жүктелуі және олардың сипаттамасы
Тағамдық қоспалардың функционалдық жүктелуі және олардың сипаттамасы Нефть. Свойства нефти
Нефть. Свойства нефти Properties of Atoms and the Periodic Table
Properties of Atoms and the Periodic Table Функциональные производные с кратной связью C=“Э”. Часть 1. Карбонильные соединения и имины
Функциональные производные с кратной связью C=“Э”. Часть 1. Карбонильные соединения и имины Периодический закон и периодическая система Д.И. Менделеева. Вторичная периодичность. Тема №1
Периодический закон и периодическая система Д.И. Менделеева. Вторичная периодичность. Тема №1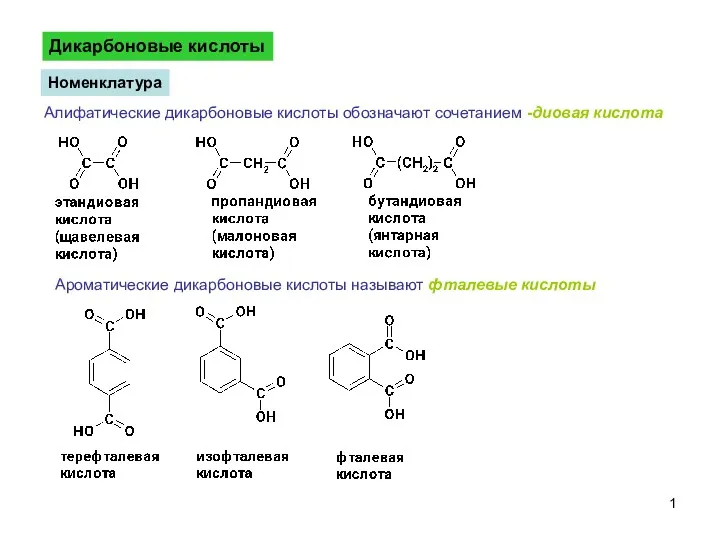 ДикарбоновыеКислоты-1
ДикарбоновыеКислоты-1 История органической химии. Характерные особенности органических соединений
История органической химии. Характерные особенности органических соединений Спирти. Насичені одноатомні спирти: формули, ізомерія, систематична номенклатура
Спирти. Насичені одноатомні спирти: формули, ізомерія, систематична номенклатура Стекло. Виды стекол
Стекло. Виды стекол Реакции ионного обмена
Реакции ионного обмена Соединения галогенов
Соединения галогенов Химический элемент. Неон
Химический элемент. Неон Алкины
Алкины Спирты. Обобщающий урок
Спирты. Обобщающий урок Химия өнеркәсібі
Химия өнеркәсібі Современные представления о катализе. Общие закономерности гетерогенного катализа
Современные представления о катализе. Общие закономерности гетерогенного катализа Organic chemistry. Alcohols
Organic chemistry. Alcohols Классификация химических элементов
Классификация химических элементов Строение атома. Периодическая таблица Менделеева. Химическая связь
Строение атома. Периодическая таблица Менделеева. Химическая связь Основные положения теории растворов электролитов, используемых в аналитической химии
Основные положения теории растворов электролитов, используемых в аналитической химии Соединения железа
Соединения железа Типы химических реакций в органической химии
Типы химических реакций в органической химии Синтез высокомолекулярных соединений
Синтез высокомолекулярных соединений Химические методы выявления потожировых следов
Химические методы выявления потожировых следов Природные каменные материалы. (Лекция 3)
Природные каменные материалы. (Лекция 3) Правила безопасности в кабинете химии. Приёмы обращения с лабораторным оборудованием
Правила безопасности в кабинете химии. Приёмы обращения с лабораторным оборудованием